��Ŀ����

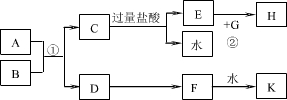
��1��B��D��E�Ļ�ѧʽ����Ϊ_________��_________��__________��
��2��д��B��������Һ��˫��ˮ��Ӧ�����ӷ���ʽ��____________________��
��3����һ�������£���A���ȵĹ���Ũ���ᷴӦ�����壬ͨ��D�У��ɷ�����ѧ��Ӧ��д���÷�Ӧ�����ӷ���ʽ______________��
��4��д�������ʵ�����B�������Ʒ�Ӧ����F�Ļ�ѧ����ʽ_____________������������������������������
��2��2Fe2++H2O2+2H+==2Fe3++2H2O
��3��2Fe3++SO2+2H2O== 2Fe2++SO42-+4H+
��4��4Na2O2+6H2O+4FeSO4==4Fe(OH)3��+4Na2SO4+O2��

��ѧ��ѧ�м��ֳ������ʵ�ת����ϵ��ͼ��ʾ(ͼ�в��ַ�Ӧ��������P��Ӧ����δ�г�)��
 |
��֪��A��B��C��D��E�ǵ��ʣ�������ǵ�Ԫ�ص�ԭ����������Ϊa��b��c��d��e����3(a + b) = 2(a + c) = 3(d ��a)��X��Y��Z��M��N��W��H��K�ǻ��������X��B��C�Ļ��ϲ����ˮ��Һ����ش��������⣺
��1���õ���ʽ��ʾX�����ʵ��γɹ��̣�_______________�����C��Ԫ�ص�ԭ�ӽṹʾ��ͼ��_____ ��
��2��д��B��������ȼ�����ɵIJ�����H2O��Ӧ�Ļ�ѧ����ʽ��_______________��
��3����ɵ���B��C��D������Ԫ�ؼ����ӵ����Ӱ뾶�ɴ�С��˳����__ _(�����ӷ��ű�ʾ)��
��4��д��K������İ�ˮ��Ӧ�Ļ�ѧ����ʽ ��д��M��ˮ��Һ�е���ķ���ʽ ��
��ѧ��ѧ�м��ֳ������ʵ�ת����ϵ����ͼ��ʾ�����ַ�Ӧ������P��Ӧ��������ȥ����
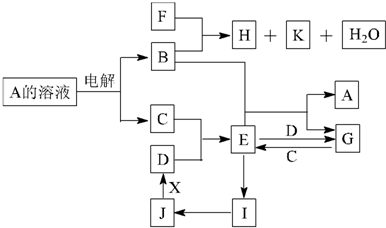
��֪��B��C��DΪ�������ʣ�����CΪ���壬B��DΪ������FΪ������ǿ�K������Ϊ���壬Ŀ��ʹƷ����Һ��ɫ���� E ��Һ�����ˮ���Ƶ�һ�ֺ��ɫ���壻 J Ϊ����ɫ���塣��ش��������⣺
 |
( l ) B �Ļ�ѧʽΪ ��
д�� B �� F ��Ӧ�Ļ�ѧ����ʽ
( 2��ʵ���б��� G ��ҺʱҪ���� Ŀ����
( 3��Ϊʵ��JһD�ı仯����X�Ƿǽ������ʣ���X������ ��д��ѧʽ��; ��X�ǽ������ʣ���д�� J һ D ��Ӧ�Ļ�ѧ����ʽ