��Ŀ����
����˵����ȷ����
- A.��100mL3mol?L-1��H2SO4��100mLH2O��ϣ���������ʵ���Ũ�ȸı�Ϊ1.5mol?L-1
- B.��200mL3mol?L-1��BaCl2��Һ��100mL3mol?L-1��KCl��Һ��Ϻ���Һ�е�C��Cl-����Ȼ��3mol?L-1
- C.��100g20%��NaCl��Һ��100gH2O��Ϻ�NaCl��Һ������������10%
- D.��100mL20%��NaOH��Һ��100mLH2O��Ϻ�NaOH��Һ������������10%
C
������A��ϡ��ǰ��������������ʵ������䣬�����Һ���С��200ml��
B��3mol?L-1��BaCl2��Һ��������Ũ��Ϊ6mol/L��3mol?L-1��KCl��Һ��������Ũ��Ϊ3molL��������Ũ�Ƚ���3mol/L��6mol/L��
C����Ϻ���Һ����Ϊ200g�������Ȼ��Ƶ���������Ϊ100g��20%=20���������������Ķ�����㣮
D��ˮ���ܶ�С������������Һ�ܶȣ���Ϻ���Һ������С��2����100mL20%������������Һ������
���A��ϡ��ǰ��������������ʵ������䣬�����Һ���С��200ml������ϡ�ͺ���������ʵ���Ũ�ȴ���Ϊ
1.5mol?L-1����A����
B��3mol?L-1��BaCl2��Һ��������Ũ��Ϊ6mol/L��3mol?L-1��KCl��Һ��������Ũ��Ϊ3molL��������Ũ�Ƚ���3mol/L��6mol/L������������仯����Ϻ�������Ũ��ԼΪ =5mol/L����B����
=5mol/L����B����
C����Ϻ���Һ����Ϊ200g�������Ȼ��Ƶ���������Ϊ100g��20%=20g�����Ի�Ϻ�����������Һ��������Ϊ
 ��100%=10%����C��ȷ��
��100%=10%����C��ȷ��
D��ˮ���ܶ�С������������Һ�ܶȣ���Ϻ���Һ������С��2����100mL20%������������Һ���������Ի�Ϻ������������Һ��������������10%����D����
��ѡ��C��
�������������ʵ���Ũ�ȡ����������ļ��㣬�ѶȲ��ؼ������Һ�ܶȶ���Һ�����������Ӱ�죮
������A��ϡ��ǰ��������������ʵ������䣬�����Һ���С��200ml��
B��3mol?L-1��BaCl2��Һ��������Ũ��Ϊ6mol/L��3mol?L-1��KCl��Һ��������Ũ��Ϊ3molL��������Ũ�Ƚ���3mol/L��6mol/L��
C����Ϻ���Һ����Ϊ200g�������Ȼ��Ƶ���������Ϊ100g��20%=20���������������Ķ�����㣮
D��ˮ���ܶ�С������������Һ�ܶȣ���Ϻ���Һ������С��2����100mL20%������������Һ������
���A��ϡ��ǰ��������������ʵ������䣬�����Һ���С��200ml������ϡ�ͺ���������ʵ���Ũ�ȴ���Ϊ
1.5mol?L-1����A����
B��3mol?L-1��BaCl2��Һ��������Ũ��Ϊ6mol/L��3mol?L-1��KCl��Һ��������Ũ��Ϊ3molL��������Ũ�Ƚ���3mol/L��6mol/L������������仯����Ϻ�������Ũ��ԼΪ
 =5mol/L����B����
=5mol/L����B����C����Ϻ���Һ����Ϊ200g�������Ȼ��Ƶ���������Ϊ100g��20%=20g�����Ի�Ϻ�����������Һ��������Ϊ
 ��100%=10%����C��ȷ��
��100%=10%����C��ȷ��D��ˮ���ܶ�С������������Һ�ܶȣ���Ϻ���Һ������С��2����100mL20%������������Һ���������Ի�Ϻ������������Һ��������������10%����D����
��ѡ��C��
�������������ʵ���Ũ�ȡ����������ļ��㣬�ѶȲ��ؼ������Һ�ܶȶ���Һ�����������Ӱ�죮

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ
Ӱ�컯ѧ��Ӧ���ʵ����غܶ࣬ij������ȤС����ʵ��ķ����о���Ӧ���ʵ��й����⣮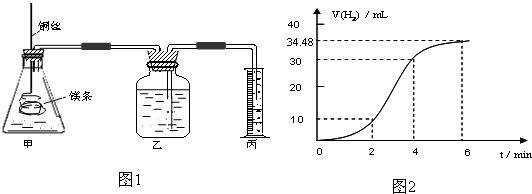
��1��ʵ��1 ̽��Mg�����ᷴӦ���ʵı仯���ɣ�ȡһ��þ������ɰֽ��ȥ���������Ĥ��ͭ˿����þ������װ�ü��У�ʹþ��������ƿ�ڵ����Ϊ2Lϡ���ᣨ�������У�þ�������ᷴӦ����H2������뷴Ӧʱ��Ĺ�ϵ������ͼ2��ʾ��
�ٴ�ͼ2�п���0-6min��ƽ����Ӧ��������ʱ����� ��������ţ�
A��0-2min B��2-4min C��4-6min
�������4-6min ʱ���ڣ���HCl��ʾ��ƽ����Ӧ����Ϊ ��������ͼ2����������ѻ���Ϊ��״���µ����������Һ����仯�ɺ��ԣ�
��ͼ1װ�ü�����þ��������ͭ˿��һ�����ϡ�����жԷ�Ӧ����Ӱ������˵����ȷ����
A���ӿ췴Ӧ���ʵ������������������� B��������Ӧ������������������
C����Ӱ�췴Ӧ���� D���ӿ췴Ӧ���ʵ�����������������С
��2��ʵ��2 ̽����Ũ�ȶ�MnO2��H2O2��Ӧ���ʵ�Ӱ��
��֪MnO2+H2O2+2H+�TMn2++O2��+2H2O����ȡ����MnO2���±��й����ʣ�����ͬ�¶��½���4��ʵ�飬�ֱ��¼�ռ�20.0mL��������ʱ�䣮
���ϱ���V1= mL��V3= mL��
����ͬѧ���ʵ��I������Ϊʵ����ĶԱ�ʵ�飬�������� ��
����ʵ����t2��t3��t4����ɵó���ʵ������� ��

��1��ʵ��1 ̽��Mg�����ᷴӦ���ʵı仯���ɣ�ȡһ��þ������ɰֽ��ȥ���������Ĥ��ͭ˿����þ������װ�ü��У�ʹþ��������ƿ�ڵ����Ϊ2Lϡ���ᣨ�������У�þ�������ᷴӦ����H2������뷴Ӧʱ��Ĺ�ϵ������ͼ2��ʾ��
�ٴ�ͼ2�п���0-6min��ƽ����Ӧ��������ʱ�����
A��0-2min B��2-4min C��4-6min
�������4-6min ʱ���ڣ���HCl��ʾ��ƽ����Ӧ����Ϊ
��ͼ1װ�ü�����þ��������ͭ˿��һ�����ϡ�����жԷ�Ӧ����Ӱ������˵����ȷ����
A���ӿ췴Ӧ���ʵ������������������� B��������Ӧ������������������
C����Ӱ�췴Ӧ���� D���ӿ췴Ӧ���ʵ�����������������С
��2��ʵ��2 ̽����Ũ�ȶ�MnO2��H2O2��Ӧ���ʵ�Ӱ��
��֪MnO2+H2O2+2H+�TMn2++O2��+2H2O����ȡ����MnO2���±��й����ʣ�����ͬ�¶��½���4��ʵ�飬�ֱ��¼�ռ�20.0mL��������ʱ�䣮
| ʵ���� | �� | �� | �� | �� |
| 10%H2O2�����/mL | 5.0 | 5.0 | V1 | V2 |
| 20%��������/mL | 0 | 0.5 | 1.0 | V3 |
| ˮ�����/mL | 15 | 14.5 | V4 | 13.5 |
| ����ʱ��t/s | t1 | t2 | t3 | t4 |
����ͬѧ���ʵ��I������Ϊʵ����ĶԱ�ʵ�飬��������
����ʵ����t2��t3��t4����ɵó���ʵ�������


 �ǽ���Ԫ�ص��ж����������NO��NO2��N2O4�ȣ�
�ǽ���Ԫ�ص��ж����������NO��NO2��N2O4�ȣ�