��Ŀ����
 ��98%��ŨH2SO4����=1.84g/cm3������480mL0.5mol/L��ϡH2SO4���밴Ҫ����գ�
��98%��ŨH2SO4����=1.84g/cm3������480mL0.5mol/L��ϡH2SO4���밴Ҫ����գ�
��1������ŨH2SO4�����ʵ���Ũ��Ϊ______������ʱ����Ũ��������Ϊ______��
��2��ʵ������Ҫ�õ��Ķ���������______��
��3����ʵ���г������������������ҺŨ����ʲôӰ�죿����ƫ�ߡ�ƫ�͡���Ӱ�죩
��Ũ�����ܽ��δ�������¼����ж���______��
�ڶ���ʱ���ӿ̶���______��
��4��������ʱҺ����ڿ̶���Ӧ��ȡ�Ĵ�ʩ��______��
��5��������ȫ����ȷ��������õ���ҺӦ������Լ�ƿ�У������ϱ�ǩ����������ѱ�ǩ�ϵ�����дһ�£�����ͼ����
�⣺��1��ŨH2SO4�����ʵ���Ũ��c=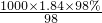 mol/L=18.4mol/L��
mol/L=18.4mol/L��
����480mL0.2mol/L��ϡH2SO4����ѡ��500mL����ƿ������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL��18.4mol/L=500mL��0.5mol/L����ã�x��13.6������Ӧ��ȡ��Ũ���������13.6mL��
�ʴ�Ϊ��18.4mol/L�� 13.6mL��
��2�����������м��㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�������20mL��Ͳ��ȡ���õ���ͷ�ιܣ�Ũ���ᣬ���ձ���ϡ�ͣ��ò��������裬��ȴ�����º�ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ���ϴ��Һת�Ƶ�����ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμ�����Һ��Һ����̶���ˮƽ���У��Ǻ�ƿ���ߵ�ҡ�ȣ�
������Ҫ������Ϊ�����������ձ�����ͷ�ιܡ�20 mL��Ͳ��500mL����ƿ��
�ʴ�Ϊ��20 mL��Ͳ��500mL����ƿ��
��3����Ũ�����ܽ��δ�������¼�����ת�ơ����ݣ�һ����ȴ�����ᵼ�����ƫС��������ҺŨ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
�ڶ���ʱ���ӿ̶��ߣ���Һ���ƫС��������ҺŨ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��4������ʱҺ����ڿ̶���Ӧ����������ҺŨ��ƫ�ͣ�Ӧ����ʵ��ʧ�ܣ�ϴ������ƿ���������ƣ��ʴ�Ϊ��Ӧ����ʵ��ʧ�ܣ�ϴ������ƿ���������ƣ�
��5����ǩ�ϵ�����Ӧд�����ʵ����ƺ�Ũ�ȣ�
�ʴ�Ϊ�� ��
��
��������1������c=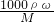 ����Ũ��������ʵ���Ũ�ȣ��ٸ�����Һϡ��ǰ���������ʵ��������������Ũ����������
����Ũ��������ʵ���Ũ�ȣ��ٸ�����Һϡ��ǰ���������ʵ��������������Ũ����������
��2������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
��3���������������ʵ����ʵ��������Һ�������Ӱ�죬����c= �����жϣ�
�����жϣ�
��4��ʵ�����������һ�����ִ���Ӧ����ʵ��ʧ�ܣ�ϴ������ƿ���������ƣ�
��5����ǩӦע�����ʵ����ƺ�Ũ�ȣ�
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ�ע���c= ��������ԭ����ע��Ũ�����ϡ�Ͳ�����
��������ԭ����ע��Ũ�����ϡ�Ͳ�����
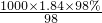 mol/L=18.4mol/L��
mol/L=18.4mol/L������480mL0.2mol/L��ϡH2SO4����ѡ��500mL����ƿ������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL��18.4mol/L=500mL��0.5mol/L����ã�x��13.6������Ӧ��ȡ��Ũ���������13.6mL��
�ʴ�Ϊ��18.4mol/L�� 13.6mL��
��2�����������м��㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�������20mL��Ͳ��ȡ���õ���ͷ�ιܣ�Ũ���ᣬ���ձ���ϡ�ͣ��ò��������裬��ȴ�����º�ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ���ϴ��Һת�Ƶ�����ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμ�����Һ��Һ����̶���ˮƽ���У��Ǻ�ƿ���ߵ�ҡ�ȣ�
������Ҫ������Ϊ�����������ձ�����ͷ�ιܡ�20 mL��Ͳ��500mL����ƿ��
�ʴ�Ϊ��20 mL��Ͳ��500mL����ƿ��
��3����Ũ�����ܽ��δ�������¼�����ת�ơ����ݣ�һ����ȴ�����ᵼ�����ƫС��������ҺŨ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
�ڶ���ʱ���ӿ̶��ߣ���Һ���ƫС��������ҺŨ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��4������ʱҺ����ڿ̶���Ӧ����������ҺŨ��ƫ�ͣ�Ӧ����ʵ��ʧ�ܣ�ϴ������ƿ���������ƣ��ʴ�Ϊ��Ӧ����ʵ��ʧ�ܣ�ϴ������ƿ���������ƣ�
��5����ǩ�ϵ�����Ӧд�����ʵ����ƺ�Ũ�ȣ�
�ʴ�Ϊ��
 ��
����������1������c=
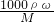 ����Ũ��������ʵ���Ũ�ȣ��ٸ�����Һϡ��ǰ���������ʵ��������������Ũ����������
����Ũ��������ʵ���Ũ�ȣ��ٸ�����Һϡ��ǰ���������ʵ��������������Ũ������������2������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
��3���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=
 �����жϣ�
�����жϣ���4��ʵ�����������һ�����ִ���Ӧ����ʵ��ʧ�ܣ�ϴ������ƿ���������ƣ�
��5����ǩӦע�����ʵ����ƺ�Ũ�ȣ�
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ�ע���c=
 ��������ԭ����ע��Ũ�����ϡ�Ͳ�����
��������ԭ����ע��Ũ�����ϡ�Ͳ����� 
��ϰ��ϵ�д�
�����Ŀ
 ��98%��ŨH2SO4����=1.84g/cm3������480mL0.5mol/L��ϡH2SO4���밴Ҫ����գ�
��98%��ŨH2SO4����=1.84g/cm3������480mL0.5mol/L��ϡH2SO4���밴Ҫ����գ� ��98%��ŨH2SO4����=1.84g/cm3������480mL0.5mol/L��ϡH2SO4���밴Ҫ����գ�
��98%��ŨH2SO4����=1.84g/cm3������480mL0.5mol/L��ϡH2SO4���밴Ҫ����գ�