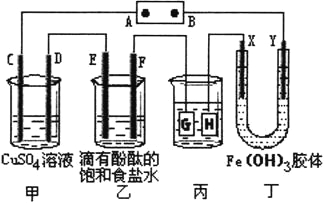
��Ŀ����
����Ŀ����ѧƽ��ԭ������ѧ��ѧѧϰ����Ҫ���ݣ���ش��������⣺
�״���һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ������ҵ�Ͽ��úϳ�������Ҫ�ɷ�CO��H2���Ʊ��״���
��1����֪��CO��H2��CH3OH����ȼ��������H���ֱ�Ϊ-283.0kJ/mol��-241.8kJ/mol��-192.2 kJ/mol����д���ϳ����Ʊ��״����Ȼ�ѧ����ʽ ��
��2�����ھ��ȡ����ݵ��ܱ������г���1 mol CO��2 mol H2������CO��g��+2H2��g��![]() CH3OH��g����Ӧ������ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ��Ϊƽ��״̬����______����ѡ����ĸ����
CH3OH��g����Ӧ������ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ��Ϊƽ��״̬����______����ѡ����ĸ����
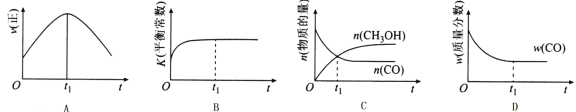
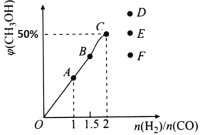
��3����T1��ʱ�������Ϊ5 L�ĺ��������г���3 mol�ĺϳ�������Ӧ�ﵽƽ��ʱCH3OH�����������n��H2����n��CO���Ĺ�ϵ��ͼ��ʾ��H2��CO��2:1Ͷ��ʱ����5 min�ﵽƽ�⣬��5 min����H2��ʾ�ķ�Ӧ����Ϊv��H2��=_______���¶Ȳ��䣬��![]() ʱ���ﵽƽ��״̬��CH3OH���������������ͼ���е�______�㡣
ʱ���ﵽƽ��״̬��CH3OH���������������ͼ���е�______�㡣
��4�����м״��ķ�ˮ�����ŷŻ����ˮ��Ⱦ������ClO2��������ΪCO2��Ȼ���ټӼ��кͼ��ɡ�д�������״����Է�ˮ�����У�ClO2��״���Ӧ�����ӷ���ʽ��________________________��
��5��ˮ����ż����ɱ�ʾΪH2O+H2O![]() H3O++OH-����ˮ�������ƣ��״�Ҳ�ܷ�����ż���룬��д���״�����ż���뷽��ʽ_______________________________________�����״��м������������Ʒ�Ӧ���ɼ״��ƣ���Ӧ��Ļ��Һ�еĵ���غ�ʽ_____________________________��
H3O++OH-����ˮ�������ƣ��״�Ҳ�ܷ�����ż���룬��д���״�����ż���뷽��ʽ_______________________________________�����״��м������������Ʒ�Ӧ���ɼ״��ƣ���Ӧ��Ļ��Һ�еĵ���غ�ʽ_____________________________��
���𰸡���1��CO��g��+2H2��g��![]() CH3OH��l�� ��H= -574.4kJ/mol ��3���� ��2��D��2����
CH3OH��l�� ��H= -574.4kJ/mol ��3���� ��2��D��2����
��3��0.06 molL-1min-1 ��2������F��2����
��4��6ClO2 +5CH3OH��5CO2 +6Cl -+6H++7H2O��2����
��5��CH3OH+CH3OH![]() CH3OH2++CH3O -��2���� c��CH3OH2+��+ c��Na+����c��CH3O -����2����
CH3OH2++CH3O -��2���� c��CH3OH2+��+ c��Na+����c��CH3O -����2����
��������
�����������1����֪��CO��H2��CH3OH����ȼ��������H���ֱ�Ϊ-283.0kJ/mol��-241.8kJ/mol��-192.2 kJ/mol������CO��g��+1/2O2��g��=CO2��g����H=-283.0kJmol-1
��2H2��g�� + O2��g�� = 2H2O��l�� ��H=��483.6kJ/mol
��CH3OH��l��+3/2O2��g��=CO2��g��+2 H2O��l����H=-192.2kJmol-1
�ɸ�˹���ɿ�֪��+��-���÷�ӦCO��g�� + 2H2��g����CH3OH��l����H����-283-483.6+192.2��kJmol-1����574.4kJ/mol��
��2��A������ƽ���������������ȣ����ٱ仯����t1ʱ��V�����֮�����ʼ�С��A����B���÷�Ӧ����ӦΪ���ȷ�Ӧ���淴Ӧ�����¶����ߣ���ѧƽ�ⳣ����С������ƽ����¶�Ϊ��ֵ������ߣ�ƽ�ⳣ�����䣬Ϊ��С��ͼ����ʵ�ʷ��ϣ�B����C��t1ʱ�̺�һ����̼���״������ʵ��������仯��t1ʱ��δ����ƽ��״̬��C����D��t1ʱ�̣�CO����������Ϊ��ֵ������ƽ��״̬��ͼ����ʵ�ʷ��ϣ�D��ȷ����ѡD��
��3������ͼ���֪H2��CO��2:1Ͷ��ƽ��ʱ�״��ĺ�����0.5������ݷ���ʽ��֪
CO��g��+2H2��g��![]() CH3OH��g��
CH3OH��g��
��ʼ����mol�� 1 2 0
ƽ������mol�� n 2n n
ת������mol��1��n 2��2n n
�����![]()
���n��0.75
����5 min����H2��ʾ�ķ�Ӧ����Ϊ![]() ��0.06 molL-1min-1��
��0.06 molL-1min-1��
�¶Ȳ��䣬��![]() ʱ���������ʵ������ӣ�CO��ת��������������ת���ʼ�С����˲����ļ״������ʵ�����С�������ﵽƽ��״̬ʱCH3OH������������������������ͼ���е�F����
ʱ���������ʵ������ӣ�CO��ת��������������ת���ʼ�С����˲����ļ״������ʵ�����С�������ﵽƽ��״̬ʱCH3OH������������������������ͼ���е�F����
��4����Ӧ��ClԪ�ػ��ϼ۴ӣ�4�����͵���1�����õ�5�����ӡ�̼Ԫ�ػ��ϼ�����2�����ߵ���4����ʧȥ6����������˸��ݵ��ӵ�ʧ�غ��֪ClO2��״���Ӧ�����ӷ���ʽΪ6ClO2 +5CH3OH��5CO2 +6Cl -+6H++7H2O��
��5������ˮ����ż����ɱ�ʾΪH2O+H2O![]() H3O++OH-��֪�״�����ż���뷽��ʽΪCH3OH��CH3OH
H3O++OH-��֪�״�����ż���뷽��ʽΪCH3OH��CH3OH![]() CH3OH2+��CH3O -�����״��м������������Ʒ�Ӧ���ɼ״��ƣ���Ӧ��Ļ��Һ�еĵ���غ�ʽΪc��CH3OH2+��+ c��Na+����c��CH3O -����
CH3OH2+��CH3O -�����״��м������������Ʒ�Ӧ���ɼ״��ƣ���Ӧ��Ļ��Һ�еĵ���غ�ʽΪc��CH3OH2+��+ c��Na+����c��CH3O -����

����Ŀ���±���Ԫ�����ڱ���һ���֡��������е���ĸ�ֱ����ijһ�ֻ�ѧԪ�ء�
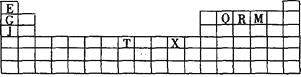

��1��д����̬T3+�ĺ�������Ų�ʽ�� ��T�����ڱ������ڷ���Ϊ ��
��2��Q��R��M�ĵ�һ�������ɴ�С��˳���� (��Ԫ�ط��ű�ʾ)��ԭ�� ��
��3�������й�����Ԫ�ص�˵����ȷ���� ��
A��J��X���ã�����J��������Һ���û���X |
B����J2M2����ˮ��Ҫ�ƻ����Ӽ����ۼ� |
C��RE3�е����QE4����Ҫ����Ϊǰ����Է��������ϴ� |
D��һ��Q2E4�����к������������һ������ |
��4��G2O���۵��J2O�� �������������������� ����ԭ���� ��
��5�� G��R����ֱ�ӻ�������һ�����ӻ�����G3R���þ����������ʯī�IJ�״�ṹ��ÿ���У�Gԭ�ӹ���ƽ�������Σ�ÿ�������ε�������һ��Rԭ�ӡ������֮�仹����һ��������ԭ�ӡ�������Щ���ӵ�ԭ��Ӧ���� (��G��R��Ԫ�ط���)��