��Ŀ����
A��B��C��D�ֱ����NH4Cl����NH4��2SO4��Ba��OH��2��Na2SO4��4����Һ�е�ijһ�֣�������������ϲ�����������
��1��A��B��ϣ�������ɫ����������ʱ�̼����������ɣ�
��2��B��C���Ҳ������ɫ������������ʱ�д̼����������ɣ��Ҹ�������ʹʪ��ĺ�ɫʯ����ֽ������
��3��B��D��ϣ��������������Ⱥ������ʹʪ��ĺ�ɫʯ����ֽ���������壮
��ش��������⣺
��1��д���������ʵĻ�ѧʽ��
A______��B______�� C______�� D______
��2��д�������йط�Ӧ��ѧ����ʽ��
A+B______��
B+C______��
B+D______��
��3�����ϻ�ѧ��Ӧ�ɹ��Ϊ�������ӷ���ʽ����д����
��______��
��______��
�⣺��1�����������������ʷ�Ӧ���ɰ�ɫ������ֻ��Ba��OH��2����BΪBa��OH��2��
A��B��ϣ�������ɫ����������ʱ�̼����������ɣ�˵��AΪNa2SO4��
B��C���Ҳ������ɫ������������ʱ�д̼����������ɣ��Ҹ�������ʹʪ��ĺ�ɫʯ����ֽ������������Ϊ��������CΪ��NH4��2SO4��
B��D��ϣ��������������Ⱥ������ʹʪ��ĺ�ɫʯ����ֽ���������壬DӦΪNH4Cl��
�ʴ�Ϊ��Na2SO4��Ba��OH��2����NH4��2SO4��NH4Cl��
��2��AΪNa2SO4��BΪBa��OH��2�����߷�Ӧ�Ļ�ѧ����ʽΪNa2SO4+Ba��OH��2=BaSO4��+2NaOH��
BΪBa��OH��2��CΪ��NH4��2SO4�����߷�Ӧ�Ļ�ѧ����ʽΪBa��OH��2+��NH4��2SO4=BaSO4��+2NH3��+2 H2O��
BΪBa��OH��2��DΪNH4Cl�����߷�Ӧ�Ļ�ѧ����ʽΪBa��OH��2+2NH4Cl=BaCl2+2NH3��+2 H2O��
�ʴ�Ϊ��Na2SO4+Ba��OH��2=BaSO4��+2NaOH��
Ba��OH��2+��NH4��2SO4=BaSO4��+2NH3��+2 H2O��
Ba��OH��2+2NH4Cl=BaCl2+2NH3��+2 H2O��
��3�����Ϸ�Ӧ�漰SO42-��Ba2+�Լ�OH-��NH4+�ķ�Ӧ����Ӧ�����ӷ���ʽ�ֱ�ΪSO42-+Ba2+=BaSO4����OH-+NH4+=NH3��+H2O��
�ʴ�Ϊ����SO42-+Ba2+=BaSO4������OH-+NH4+=NH3��+H2O��
���������������������ʷ�Ӧ���ɰ�ɫ������ֻ��Ba��OH��2����BΪBa��OH��2��
��1��A��B��ϣ�������ɫ����������ʱ�̼����������ɣ�˵��AΪNa2SO4��
��2��B��C���Ҳ������ɫ������������ʱ�д̼����������ɣ��Ҹ�������ʹʪ��ĺ�ɫʯ����ֽ������������Ϊ��������CΪ��NH4��2SO4��
��3��B��D��ϣ��������������Ⱥ������ʹʪ��ĺ�ɫʯ����ֽ���������壬DӦΪNH4Cl��
��϶�Ӧ���ʵ����ʽ����⣮
�����������ۺϿ���Ԫ�ػ�����֪ʶ�������ڿ���ѧ���ۺ����û�ѧ֪ʶ��������ע����ݷ�Ӧ����������жϣ���Ŀ�ѶȲ���
A��B��ϣ�������ɫ����������ʱ�̼����������ɣ�˵��AΪNa2SO4��
B��C���Ҳ������ɫ������������ʱ�д̼����������ɣ��Ҹ�������ʹʪ��ĺ�ɫʯ����ֽ������������Ϊ��������CΪ��NH4��2SO4��
B��D��ϣ��������������Ⱥ������ʹʪ��ĺ�ɫʯ����ֽ���������壬DӦΪNH4Cl��
�ʴ�Ϊ��Na2SO4��Ba��OH��2����NH4��2SO4��NH4Cl��
��2��AΪNa2SO4��BΪBa��OH��2�����߷�Ӧ�Ļ�ѧ����ʽΪNa2SO4+Ba��OH��2=BaSO4��+2NaOH��
BΪBa��OH��2��CΪ��NH4��2SO4�����߷�Ӧ�Ļ�ѧ����ʽΪBa��OH��2+��NH4��2SO4=BaSO4��+2NH3��+2 H2O��
BΪBa��OH��2��DΪNH4Cl�����߷�Ӧ�Ļ�ѧ����ʽΪBa��OH��2+2NH4Cl=BaCl2+2NH3��+2 H2O��
�ʴ�Ϊ��Na2SO4+Ba��OH��2=BaSO4��+2NaOH��
Ba��OH��2+��NH4��2SO4=BaSO4��+2NH3��+2 H2O��
Ba��OH��2+2NH4Cl=BaCl2+2NH3��+2 H2O��
��3�����Ϸ�Ӧ�漰SO42-��Ba2+�Լ�OH-��NH4+�ķ�Ӧ����Ӧ�����ӷ���ʽ�ֱ�ΪSO42-+Ba2+=BaSO4����OH-+NH4+=NH3��+H2O��
�ʴ�Ϊ����SO42-+Ba2+=BaSO4������OH-+NH4+=NH3��+H2O��
���������������������ʷ�Ӧ���ɰ�ɫ������ֻ��Ba��OH��2����BΪBa��OH��2��
��1��A��B��ϣ�������ɫ����������ʱ�̼����������ɣ�˵��AΪNa2SO4��
��2��B��C���Ҳ������ɫ������������ʱ�д̼����������ɣ��Ҹ�������ʹʪ��ĺ�ɫʯ����ֽ������������Ϊ��������CΪ��NH4��2SO4��
��3��B��D��ϣ��������������Ⱥ������ʹʪ��ĺ�ɫʯ����ֽ���������壬DӦΪNH4Cl��
��϶�Ӧ���ʵ����ʽ����⣮
�����������ۺϿ���Ԫ�ػ�����֪ʶ�������ڿ���ѧ���ۺ����û�ѧ֪ʶ��������ע����ݷ�Ӧ����������жϣ���Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
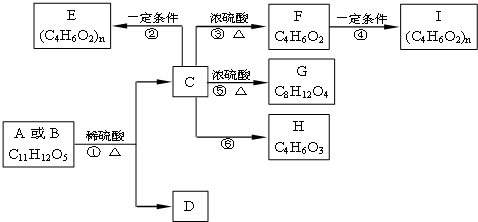
 ������A��B��C��D��E��F��G��H�����л����������ͼ��ʾ��ת����ϵ��
������A��B��C��D��E��F��G��H�����л����������ͼ��ʾ��ת����ϵ��


 ������RΪ������������A��һ������������ͼ��ʾ��ת����ϵ����֪E�������ܶ�����ͬ������H2�ܶȵ�74����������ɷ���CaHbO2��
������RΪ������������A��һ������������ͼ��ʾ��ת����ϵ����֪E�������ܶ�����ͬ������H2�ܶȵ�74����������ɷ���CaHbO2��