��Ŀ����
2��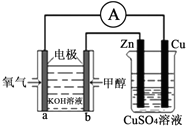 ��Դ����������������ٵ��ش���⣬�״���δ����Ҫ����Դ����֮һ�����ü״�ȼ�ϵ�������ͼ��ʾ��װ�ã�������˵���в���ȷ���ǣ�������
��Դ����������������ٵ��ش���⣬�״���δ����Ҫ����Դ����֮һ�����ü״�ȼ�ϵ�������ͼ��ʾ��װ�ã�������˵���в���ȷ���ǣ�������| A�� | ��װ����Cu��Ϊ���� | |
| B�� | ��װ�����ҳ�Ϊ��Ƴأ���������CuSO4��ҺpH���� | |
| C�� | b���ĵ缫��ӦʽΪCH3OH+8OH--6e-�TCO32-+6H2O | |
| D�� | ��ͭƬ�������仯Ϊ12.8gʱ��a�������ĵ�O2�ڱ�״���µ����Ϊ1.12L |
���� �״�ȼ�ϵ�أ�ȼ���ڸ�������������Ӧ���븺�����ӵ������������������ӵ�Ϊ��������ظ�����Ӧ�Ǽ״�ʧ���ӣ��ڼ�����Һ������̼���Σ����ݵ���غ�д���缫��Ӧ��
��� �⣺A���״�ȼ�ϵ�أ�ȼ���ڸ�������������Ӧ���븺�����ӵ������������������ӵ�Ϊ������CuΪ��������A��ȷ��
B��CuΪ������ZnΪ�������������ҺΪCuSO4��Һ�������ҳ�Ϊ��Ƴأ���������CuSO4��ҺpH���䣬��B��ȷ��
C���״�ȼ�ϵ���м״���b�缫����������Ӧ��b�缫�����ĵ缫��ӦΪ��CH3OH-6e-+8OH-=CO32-+6H2O����C��ȷ��
D����Ӧ1molCuת��2mol���ӣ���Ӧ1mol����ת��4mol���ӣ�ͭƬ�������仯Ϊ12.8gʱ��ת�Ƶ���$\frac{12.8g}{64g/mol}$��2=0.4mol��a�������ĵ�O2�ڱ�״���µ����Ϊ2.24L����D����
��ѡD��
���� ���⿼����ȼ�ϵ�ص����֪ʶ��ע������غ��Ӧ�ã���Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д�
�����Ŀ
13����ѧʵ����ѧ�����о������ص����ã��������ʵ���ͼʾ������ص�������ȷ���ǣ�������
| A�� |  �����õ�����G����ָ���ƫת����ɱȽ�Zn��Cu�Ľ����������� | |
| B�� |  �����Ҳ�С�Թ���Һ��ı仯�������ж��������������ⸯʴ | |
| C�� |  �ⶨ�к��� | |
| D�� |  ��������ƿ��������ɫ�ı仯����ˮ�б����ˮ�б�dz�������ж�2NO2��g��?N2O4��g����һ�����ȷ�Ӧ |
10�����и��������У���ѧ��������ͬ���ǣ�������
| A�� | HCl��KCl | B�� | H2S��Na2S | C�� | CH4��H2O | D�� | H2SO4��Na2SO4 |
17��2010��11��12����27�չ��ݳɹ��ٰ��˵�16�����˻ᣮ���ݲ�ȡ��һϵ�н��ܼ��š����ƻ��������Ĵ�ʩ������˵��������ǣ�������
| A�� | ��չ���ܷ��磬�Լ��ٻ������������SO2��CO2���ŷ����� | |
| B�� | ��չ��̼���ã�����̫���ܡ�����ʵ�ֳ������� | |
| C�� | ���С���������з��ö�����̼�ϳɾ�̼������ɽ������� | |
| D�� | ʹ��������շ�����δ��������������� |
7����N2+3H2 $?_{���¸�ѹ}^{����}$ 2NH3�ķ�Ӧ�У���5s��NH3��Ũ�ȱ仯��8mol/L����NH3��ƽ����Ӧ���ʣ�������
| A�� | 2.4 mol/��L•s�� | B�� | 0.8mol/��L•s�� | C�� | 1.6 mol/��L•s�� | D�� | 0.08 mol/��L•s�� |
14��������Ȼ�糣��������ˮ����������Ca3��PO4��2����ʽ���ڣ����ĵ��ʺͻ��������Ź㷺��Ӧ�ã�
��1��2P��s��+3Cl2��g���T2PCl3��g����H=-612kJ/mol
P��s��+5/2Cl2��g���TPCl5��g����H=-399kJ/mol
д��PCl5�ֽ��PCl3��Cl2���Ȼ�ѧ����ʽ��PCl5��g���TPCl3��g��+Cl2��g����H=+93kJ•mol-1��
��2��PCl5�ֽ��PCl3��Cl2�ķ�Ӧ�ǿ��淴Ӧ��T��ʱ����2.0L�����ܱ������г���1.0mol PCl5������250s�ﵽƽ�⣮��Ӧ�����вⶨ�IJ������ݼ��±���
��Ӧ��50��150s �ڵ�ƽ������v��PCl3��=1.5��10-4mol/��L•s����
��1��2P��s��+3Cl2��g���T2PCl3��g����H=-612kJ/mol
P��s��+5/2Cl2��g���TPCl5��g����H=-399kJ/mol
д��PCl5�ֽ��PCl3��Cl2���Ȼ�ѧ����ʽ��PCl5��g���TPCl3��g��+Cl2��g����H=+93kJ•mol-1��
��2��PCl5�ֽ��PCl3��Cl2�ķ�Ӧ�ǿ��淴Ӧ��T��ʱ����2.0L�����ܱ������г���1.0mol PCl5������250s�ﵽƽ�⣮��Ӧ�����вⶨ�IJ������ݼ��±���
| t/s | 0 | 50 | 150 | 250 | 350 |
| n��PCl3��/mol | 0 | 0.16 | 0.19 | 0.20 | 0.20 |
11���ִ���ѧ�ⶨ�л�����ɼ��ṹ�ķ��������϶࣮�����й�˵����ȷ���ǣ�������
| A�� | Ԫ�ط����Dz������Բ���������������Ԫ�ؼ������������Բⶨ����ӵĿռ�ṹ | |
| B�� |  �ĺ˴Ź����������������ֵ1��2��2��3 �ĺ˴Ź����������������ֵ1��2��2��3 | |
| C�� | ͨ�������߹���ͼ�������������Ҵ����������� | |
| D�� | ������������������ڲⶨ�л�����ɺͽṹ���ִ��������� |


 ��
��  ���������������=������ͬ��
���������������=������ͬ��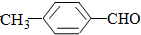 ��
��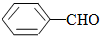
 ��CH3COOH����ʾ����ȷ��봼�����ԣ�
��CH3COOH����ʾ����ȷ��봼�����ԣ�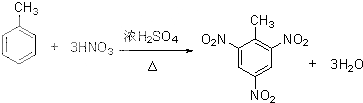 ��
�� +nHCHO
+nHCHO 
 +nH2O��
+nH2O��