��Ŀ����
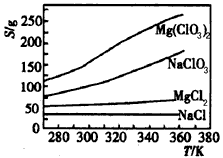
B��Mg(ClO3)2�������ȼ���S��P4��һ����
C���ɲ����ܽ⡢���˵ķ�������Mg(ClO3)2��NaCl�Ļ����
D��ͨ�����ַ����Ƶõ�Mg(ClO3)2�г�����NaCl������

 �Ķ��쳵ϵ�д�
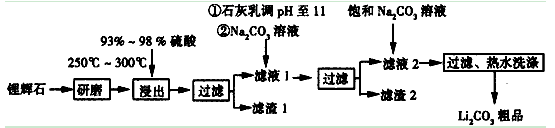
�Ķ��쳵ϵ�д�����12�֣�������ҵ����﮻�ʯ��Li2O��Al2O3��4SiO2��������Ca��MgԪ�أ�Ϊԭ������̼��ﮡ��䲿�ֹ����������£�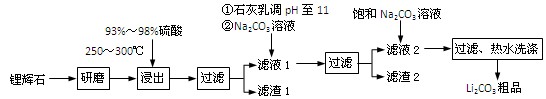
������֪���� Li2O��Al2O3��4SiO2 H2SO4��Ũ��
H2SO4��Ũ�� Li2SO4
Li2SO4 Al2O3��4SiO2��H2O��
Al2O3��4SiO2��H2O��
������ ijЩ���ʵ��ܽ��(S)���±���ʾ��
| T/�� | 20 | 40 | 60 | 80 |
 (Li2CO3)/g (Li2CO3)/g | 1.33 | 1.17 | 1.01 | 0.85 |
 (Li2SO4)/g (Li2SO4)/g | 34.2 | 32.8 | 31.9 | 30.7 |

��1����������д���������ڼ����Լ��Ļ�ѧʽ������������������������������������
��2����������д�����������õ����ʵĻ�ѧʽ������������������������������������
��3����������з�Ӧ�����ӷ���ʽ��������������������������������������
��4������֪����2����Ҫ�ɷ���Mg(OH)2��CaCO3������Һ1�м���ʯ����������ǣ����û�ѧƽ��ԭ����������������������������������������������������������������������
��5��������Һ2�м��뱥��Na2CO3��Һ�����˺��á���ˮϴ�ӡ���ԭ����������������������
��������������������������������������������������������
��6�����������ڹ�ҵ�����������ͻ���ϣ����ͻ�ש�����������������챦ʯ�ȣ�ͬʱ������Ҳ��������ԭ�ϡ�д���������������Ļ�ѧ����ʽ��������������������������������������
��ҵ����﮻�ʯ(Li2O��Al2O3��4SiO2��������Ca��MgԪ��)Ϊԭ������̼��ﮡ��䲿�ֹ����������£�
��֪����Li2O��Al2O3��4SiO2 H2SO4(Ũ)
H2SO4(Ũ)  Li2SO4
Li2SO4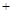 Al2O3��4SiO2��H2O��
Al2O3��4SiO2��H2O��
��ijЩ���ʵ��ܽ��(S)���±���ʾ����
|
T/�� |
20 |
40 |
60 |
80 |
|
|
1.33 |
1.17 |
1.01 |
0.85 |
|
|
34.2 |
32.8 |
31.9 |
30.7 |
�۴�����1����ȡ��Si�IJ�����������ͼ��ʾ��

����������Ϣ����ش��������⣺
��1��������Ӧ�ṩ����Ӧ�������������� �������� �� ��������
��2���������������ʵĻ�ѧʽ���� ���� ����
��3��ʹ��Ũ���Ტ���ȵ�250�桫300���Ŀ�������������������� ������
��4���ڴ���Na2CO3��Һ��������Ӧ�����ӷ���ʽ������ �� ���� ���� ��
��5������2����Ҫ�ɷ������������� ��������
��6����μ���Li2CO3�Ƿ�ϴ�Ӹɾ����������� ��������������
����12�֣�������ҵ����﮻�ʯ��Li2O��Al2O3��4SiO2��������Ca��MgԪ�أ�Ϊԭ������̼��ﮡ��䲿�ֹ����������£�
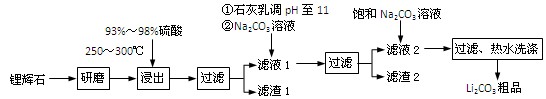
������֪���� Li2O��Al2O3��4SiO2 H2SO4��Ũ��
H2SO4��Ũ�� Li2SO4
Li2SO4 Al2O3��4SiO2��H2O��
Al2O3��4SiO2��H2O��
������ ijЩ���ʵ��ܽ��(S)���±���ʾ��
|
T/�� |
20 |
40 |
60 |
80 |
|
|
1.33 |
1.17 |
1.01 |
0.85 |
|
|
34.2 |
32.8 |
31.9 |
30.7 |
������ ������1�з����Al2O3�IJ�����������ͼ��ʾ��

��1����������д���������ڼ����Լ��Ļ�ѧʽ������������������������������������
��2����������д�����������õ����ʵĻ�ѧʽ������������������������������������
��3����������з�Ӧ�����ӷ���ʽ��������������������������������������
��4������֪����2����Ҫ�ɷ���Mg(OH)2��CaCO3������Һ1�м���ʯ����������ǣ����û�ѧƽ��ԭ����������������������������������������������������������������������
��5��������Һ2�м��뱥��Na2CO3��Һ�����˺��á���ˮϴ�ӡ���ԭ����������������������
��������������������������������������������������������
��6�����������ڹ�ҵ�����������ͻ���ϣ����ͻ�ש�����������������챦ʯ�ȣ�ͬʱ������Ҳ��������ԭ�ϡ�д���������������Ļ�ѧ����ʽ��������������������������������������
 (Li2CO3)/g
(Li2CO3)/g (Li2CO3)/g
(Li2CO3)/g
 Li2SO4+Al2O3��4SiO2��H2O��
Li2SO4+Al2O3��4SiO2��H2O�� 