��Ŀ����
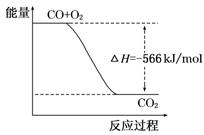
��֪��2CO(g)+O2(g)=2CO2(g) ��H=-566 kJ/mol

Na2O2(s)+CO2(g)=Na2CO3(s)+ ![]() ��H=-226 kJ/mol
��H=-226 kJ/mol
���������Ȼ�ѧ����ʽ�жϣ�����˵����ȷ���ǣ� ��
A��CO��ȼ����Ϊ283 kJ
B����ͼ�ɱ�ʾ��CO����CO2�ķ�Ӧ���̺�������
C��2Na2O2(s)+2CO2(s)=2Na2CO3(s)+O2(g) ��H��-452 kJ/mo
D��CO(g)��Na2O2(s)��Ӧ�ų�509 kJ����ʱ������ת����Ϊ6.02��1023
C
����:
A�ȼ���ȵĵ�λ������ӦΪkJ/mol������ͼ�е�����������Ӧ��Ϊ2molCO��2molCO2,�ʴ���CO2������������ڹ������������C���зų�������ӦС��452kJ������H�ø�ֵ��ʾʱ�������-452Kj/mol����ȷ������ʽ����2��Ȼ������ʽ��ӣ��ٳ���2������CO��Na2O2�ķ�Ӧ�ȣ���������Ϊ57kJ����D�����

��ϰ��ϵ�д�
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
�����Ŀ