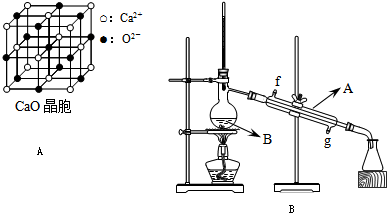
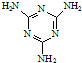
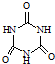
��Ŀ����
��ѡ���⣬10�֣���ij��̬����̼85.7%������14.3%���ڱ�״���µ��ܶ���2.5 g/L��������ʹ���Ը��������Һ����ˮ��ɫ��
(1)��������ʽΪ_______________________��
(2)д�����ĸ���ͬ���칹��Ľṹ��ʽ�� __________________________________��
��44.8 gͭ��140 mLһ��Ũ�ȵ����ᷴӦ��ͭ��ȫ�ܽ⣬������NO��NO2��������ڱ�״���µ����Ϊ11.2L��ش�
(1)����£�NO2�����Ϊ L
(2)������������ȫ���ͷź�����Һ�м���V mL a mol/L��NaOH��Һ��ǡ��ʹ��Һ�е�Cu2+ȫ��ת���ɳ�������ԭ������ҺŨ��Ϊ �������ʽ��
(3)��ʹͭ�����ᷴӦ���ɵ�������O2��H2Oȫ��ת��ΪHNO3������ҪO2 g
26����10�֣���ÿ��1�֣���ÿ��2�֢� (1)C4H8 (2) CH2=CHCH2CH3 CH3CH=CHCH3 CH2=C(CH3)2
��1��1.12 (2)
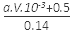 ��3��11.2
�� ��2�֣�
��3��11.2
�� ��2�֣�
���������������֪������ʹ���Ը��������Һ����ˮ��ɫ��˫����C:H=1:2.M=56g�Mmol,��������ʽΪC4H8 , ���ĸ���ͬ���칹��Ľṹ��ʽ: CH2=CHCH2CH3 CH3CH=CHCH3 CH2=C(CH3)2.
Cu+4HNO3Ũ=Cu(NO3)2+NO2��+2H2O 3Cu+8HNO3Ũ=3Cu(NO3)2+2NO��+4H2O
x x 1.5y y
64(x+1.5y)= 44.8 g x+y=0.5mol x= 0.1mol y=0.4mol
NaOH---------HNO3--------0.5 Cu2+
10-3av 0.7mol n(N)= 10-3av +0.5

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�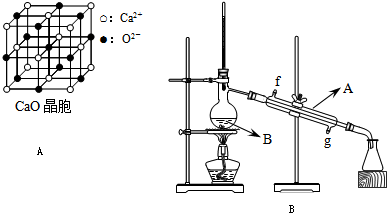

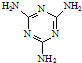 ���׳ơ����������������������谷���������ᣨ
���׳ơ����������������������谷���������ᣨ 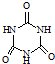 �������������������谷�����֮��ͨ��
�������������������谷�����֮��ͨ�� )����д����502�������������õĻ�ѧ����ʽ��_____________________________����Ӧ������_____________��
)����д����502�������������õĻ�ѧ����ʽ��_____________________________����Ӧ������_____________�� )Ҳ��һ���ϼ�����ҵ���ñ�ϩ���ij������һ�������·�Ӧ���Ƶ������ϼ��������ʵ�������_____________����д����һ��ȡ���̵Ļ�ѧ����ʽ_________________��
)Ҳ��һ���ϼ�����ҵ���ñ�ϩ���ij������һ�������·�Ӧ���Ƶ������ϼ��������ʵ�������_____________����д����һ��ȡ���̵Ļ�ѧ����ʽ_________________�� ��
�� ��
�� ����֪����
����֪����