��Ŀ����
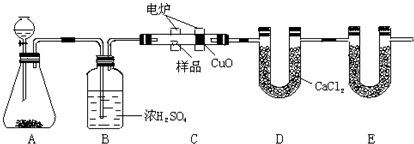
��14�֣���ѧ�ϳ���ȼ�շ�ȷ���л������ɡ���ͼװ������ȼ�շ�ȷ���������ĺ������������ʽ�ij���װ�ã����ַ������ڵ�¯����ʱ�ô�������������Ʒ�����ݲ��������ȷ���л������ɡ�

�ش��������⣺
��1��װ��A��װ��MnO2��д��Aװ���з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2��Cװ��(ȼ�չ�)��CuO�������� ��
��3��д��Eװ������ʢ�����ʵ����� ������������ ��
��4������Bװ��ȥ�����ʵ�������ʲôӰ��? ��
��5����ȷ��ȡ1.20 g��Ʒ(�������ĺ���������)�������ȼ�պ�E����������1.76 g��D����������0.72 g������л����ʵ��ʽΪ ��
��6���Ӷ����ⶨȷ�Ƕ�ȥ���ǣ���װ��Ӧ������һ���Ľ�
��
(ÿ��2�֣���14�֣�
(1) 2H2O22H2O��O2��
��2��ʹ�л�������������CO2��H2O
��3����ʯ�һ��������� ����CO2����
��4����ɲ���л����к�����ƫ��
��5��CH2O
��6����E������һ������ܣ���ֹ�����е�CO2��H2O����E��
����:

 53���ò�ϵ�д�
53���ò�ϵ�д�