��Ŀ����
A��B��C��D������ѧ��ѧ���������ʣ�����A��B��C������ͬһ��Ԫ�ء���һ���������ת���Ĺ�ϵ��ͼ��ʾ(���ַ�Ӧ�е�H2O����ȥ)���밴Ҫ��ش��������⣺

��1����DΪ�������ʣ���D�����������;���Ľ���������������B����Һû�еõ�B���Σ���B�Ļ�ѧʽ����Ϊ__________________________��
��2����A�������������B��DΪ��������Ҫ�ɷ֣���Ӧ(��) �Ļ�ѧ����ʽΪ______________��
��3����DΪ�ȼҵ����Ҫ��Ʒ����Ӧ(��)�����ӷ���ʽ������_________________________��
��4����DΪ����������壬��A��B��C��D������________________(�밴˳��д�������Ĵ�)��

��1����DΪ�������ʣ���D�����������;���Ľ���������������B����Һû�еõ�B���Σ���B�Ļ�ѧʽ����Ϊ__________________________��
��2����A�������������B��DΪ��������Ҫ�ɷ֣���Ӧ(��) �Ļ�ѧ����ʽΪ______________��
��3����DΪ�ȼҵ����Ҫ��Ʒ����Ӧ(��)�����ӷ���ʽ������_________________________��
��4����DΪ����������壬��A��B��C��D������________________(�밴˳��д�������Ĵ�)��
��1��FeCl3��Fe(NO3)3
��2��4NH3+6NO 5N2+6H2O
5N2+6H2O
��3��Al3++3AlO2-+6H2O====4Al(OH)3��
��4��NaOH��Na2CO3��NaHCO3��CO2(��NaOH��Na2SO3��NaHSO3��SO2)(������������)
��2��4NH3+6NO
 5N2+6H2O
5N2+6H2O��3��Al3++3AlO2-+6H2O====4Al(OH)3��
��4��NaOH��Na2CO3��NaHCO3��CO2(��NaOH��Na2SO3��NaHSO3��SO2)(������������)
�����������1����������֪��D�����������;���Ľ�������DΪ�����ʣ����б�ۣ�������Ԫ�ص�B������Ӧ����C��˵��B����Ԫ��Ϊ��3�ۣ�C����Ԫ��Ϊ��2�ۣ�A��������Ϊ��3�ۣ�ͬʱAҲ�ܽ�����2������C����Ϊ��3�ۣ���������B����Һû�еõ�B���Σ�˵�����ζ�Ӧ����Ϊ�ӷ����ᣬ����ƶ�AΪ����(��HNO3)��BΪ�Ȼ����ۻ�Fe(NO3)3�ݣ�CΪ�Ȼ������ۻ�Fe(NO3)2�ݣ���2����A�������������A�ǰ�����B��DΪ��������Ҫ�ɷ֣�D��������B�ǵ�����CΪNO����Ӧ(��)Ϊ������һ��������Ӧ���ɵ�����ˮ����ѧ����ʽΪ4NH3+6NO
 5N2+6H2O����3����DΪ�ȼҵ����Ҫ��Ʒ����DӦΪNaOH��������ת����ϵ֪��AΪ���������Σ�BΪ��������������CΪƫ�����Σ���Ӧ�������ӷ���ʽΪAl3++3AlO2-+6H2O=4Al��OH��3������4����DΪ����������壬A�����������巴Ӧ�������ֲ�ͬ�Ļ������A��B��C��D����NaOH��Na2CO3��NaHCO3��CO2��NaOH��Na2SO3��NaHSO3��SO2��Fe��Fe��NO3��2��Fe��NO3��3��HNO3��
5N2+6H2O����3����DΪ�ȼҵ����Ҫ��Ʒ����DӦΪNaOH��������ת����ϵ֪��AΪ���������Σ�BΪ��������������CΪƫ�����Σ���Ӧ�������ӷ���ʽΪAl3++3AlO2-+6H2O=4Al��OH��3������4����DΪ����������壬A�����������巴Ӧ�������ֲ�ͬ�Ļ������A��B��C��D����NaOH��Na2CO3��NaHCO3��CO2��NaOH��Na2SO3��NaHSO3��SO2��Fe��Fe��NO3��2��Fe��NO3��3��HNO3��
��ϰ��ϵ�д�
 Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�
�����Ŀ

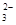 = 2I����S4O
= 2I����S4O