��Ŀ����
�״���CH3OH���Ͷ����ѣ�CH3OCH3������Ϊ21���͵�����ȼ�ϣ���CH4��H2OΪԭ���Ʊ������Ѻͼ״��Ĺ�ҵ�������£�
��1��д������Ӧ��1����һ�������½��еĻ�ѧ��Ӧ����ʽ�� ��
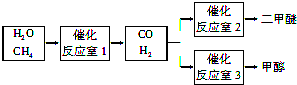
��2����ѹǿΪ0.1MPa�����£���Ӧ��3���ݻ�ΪV L����a mol CO��2a mol H2�ڴ��������·�Ӧ���ɼ״���CO��g��+2H2��g��?CH3OH��g����CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ1��ʾ����
��p1 ���������������=����p2��
���������������������£���Ӧ��3������a mol CO��2a mol H2���ﵽ��ƽ��ʱ��CO��ת���� ���������С�����䡱����
����p1ѹǿ�£�100��ʱ����Ӧ��CH3OH��g��?CO��g��+2H2��g����ƽ�ⳣ��Ϊ �����ú�a��V�Ĵ���ʽ��ʾ��
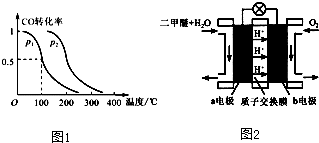
��3��ͼ2Ϊ��ɫ��Դ��������ȼ�ϵ�ء��Ĺ���ԭ��ʾ��ͼ��a�缫�ĵ缫��ӦʽΪ ��
��4��ˮú���ϳɶ����ѵ�������Ӧ���£�
��2H2��g��+CO��g��?CH3OH��g������H=-90.8kJ?mol-1
��2CH3OH��g��?CH3OCH3��g��+H2O��g������H=-23.5kJ?mol-1
��CO��g��+H2O��g��?CO2��g��+H2��g������H=-41.3kJ?mol-1
��Ӧ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g���ġ�H= ��

��1��д������Ӧ��1����һ�������½��еĻ�ѧ��Ӧ����ʽ��
��2����ѹǿΪ0.1MPa�����£���Ӧ��3���ݻ�ΪV L����a mol CO��2a mol H2�ڴ��������·�Ӧ���ɼ״���CO��g��+2H2��g��?CH3OH��g����CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ1��ʾ����
��p1
���������������������£���Ӧ��3������a mol CO��2a mol H2���ﵽ��ƽ��ʱ��CO��ת����
����p1ѹǿ�£�100��ʱ����Ӧ��CH3OH��g��?CO��g��+2H2��g����ƽ�ⳣ��Ϊ

��3��ͼ2Ϊ��ɫ��Դ��������ȼ�ϵ�ء��Ĺ���ԭ��ʾ��ͼ��a�缫�ĵ缫��ӦʽΪ
��4��ˮú���ϳɶ����ѵ�������Ӧ���£�
��2H2��g��+CO��g��?CH3OH��g������H=-90.8kJ?mol-1
��2CH3OH��g��?CH3OCH3��g��+H2O��g������H=-23.5kJ?mol-1
��CO��g��+H2O��g��?CO2��g��+H2��g������H=-41.3kJ?mol-1
��Ӧ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g���ġ�H=
��������1���ɹ�������ͼ��֪������Ӧ��1�з����ķ�Ӧ�Ǽ�����ˮ��һ������������һ����̼��������
��2������ͼ1��֪���¶���ͬʱ����ѹǿΪP2ʱƽ��ʱCO��ת���ʸߣ��ɷ�ӦCO��g��+2H2��g��?CH3OH��g����֪ѹǿԽ��Խ������ƽ��������Ӧ���У�
���������������������£���Ӧ��3������a molCO��2amolH2����ЧΪ����ѹǿ��ƽ��������Ӧ�ƶ���COת��������
����ͼ1��֪����P1ѹǿ�£�100��ʱ��CO��ת����Ϊ0.5���ݴ˼���CO��Ũ�ȱ仯������������ʽ����ƽ��ʱ����ֵ�ƽ��Ũ�ȣ�����ϳɼ״��Ļ�ѧƽ�ⳣ������������ͬ�¶��£���ͬһ��Ӧ�����淴Ӧƽ�ⳣ����Ϊ��������״��ֽ�Ļ�ѧƽ�ⳣ����
��3����Ӧ�����Ƕ����ѵ�ȼ�գ�ԭ��ظ�������������Ӧ���������ڸ����ŵ磬��ͼ��֪��a��Ϊ�����������ѷŵ����ɶ�����̼�������ӽ���Ĥ��֪�����������ӣ�
��4�����ݸ�˹��������֪���Ȼ�ѧ����ʽ������Ӧ����ֵ���мӼ���������Ŀ���Ȼ�ѧ����ʽ����Ӧ��Ҳ������Ӧ����ֵ���мӼ���
��2������ͼ1��֪���¶���ͬʱ����ѹǿΪP2ʱƽ��ʱCO��ת���ʸߣ��ɷ�ӦCO��g��+2H2��g��?CH3OH��g����֪ѹǿԽ��Խ������ƽ��������Ӧ���У�
���������������������£���Ӧ��3������a molCO��2amolH2����ЧΪ����ѹǿ��ƽ��������Ӧ�ƶ���COת��������
����ͼ1��֪����P1ѹǿ�£�100��ʱ��CO��ת����Ϊ0.5���ݴ˼���CO��Ũ�ȱ仯������������ʽ����ƽ��ʱ����ֵ�ƽ��Ũ�ȣ�����ϳɼ״��Ļ�ѧƽ�ⳣ������������ͬ�¶��£���ͬһ��Ӧ�����淴Ӧƽ�ⳣ����Ϊ��������״��ֽ�Ļ�ѧƽ�ⳣ����
��3����Ӧ�����Ƕ����ѵ�ȼ�գ�ԭ��ظ�������������Ӧ���������ڸ����ŵ磬��ͼ��֪��a��Ϊ�����������ѷŵ����ɶ�����̼�������ӽ���Ĥ��֪�����������ӣ�
��4�����ݸ�˹��������֪���Ȼ�ѧ����ʽ������Ӧ����ֵ���мӼ���������Ŀ���Ȼ�ѧ����ʽ����Ӧ��Ҳ������Ӧ����ֵ���мӼ���
����⣺��1���ɹ�������ͼ��֪������Ӧ��1�з����ķ�Ӧ�Ǽ�����ˮ��һ������������һ����̼����������Ӧ����ʽΪ��CH4+H2O
CO+3H2��
�ʴ�Ϊ��CH4+H2O
CO+3H2��
��2��������ͼ1��֪���¶���ͬʱ����ѹǿΪP2ʱƽ��ʱCO��ת���ʸߣ��ɷ�ӦCO��g��+2H2��g��?CH3OH��g����֪ѹǿԽ��Խ������ƽ��������Ӧ���У���ѹǿP1��P2��
�ʴ�Ϊ������
���������������������£���Ӧ��3������a molCO��2amolH2����ЧΪ����ѹǿ��ƽ��������Ӧ�ƶ���COת�������ʴ�Ϊ������
��CO����ʼŨ��Ϊ
mol/L��H2����ʼŨ��Ϊ
mol/L����ͼ1��֪����P1ѹǿ�£�100��ʱ��CO��ת����Ϊ0.5��CO��Ũ�ȱ仯��Ϊ
mol/L��0.5=
mol/L����
CO��g��+2H2��g��?CH3OH��g��
��ʼ��mol/L����
0
�仯��mol/L����
ƽ�⣨mol/L����
����ƽ�ⳣ��k=
=
����ͬ�¶��£�CH3OH��g��?CO��g��+2H2��g����ƽ�ⳣ��Ϊ
=
��
�ʴ�Ϊ��
��
��3����Ӧ�����Ƕ����ѵ�ȼ�գ�ԭ��ظ�������������Ӧ���������ڸ����ŵ磬��ͼ��֪��a��Ϊ�����������ѷŵ����ɶ�����̼�������ӽ���Ĥ��֪�����������ӣ�a�缫�ĵ缫��ӦʽΪ��CH3OCH3-12e-+3H2O=2CO2+12H+���ʴ�Ϊ��CH3OCH3+3H2O-12e-=2CO2+12H+��
��4����֪����CO��g��+2H2��g��?CH3OH��g����H=-90.8kJ/mol��
��2CH3OH��g��?CH3OCH3��g��+H2O��g����H=-23.5kJ/mol��
��CO��g��+H2O��g��?CO2��g��+H2��g����H=-41.3kJ/mol��
�ɸ�˹���ɿ�֪���١�2+��+�۵�3CO��g��+3H2��g��?CH3OCH3��g��+CO2��g����H=-246.4kJ/mol��
�ʴ�Ϊ��-246.4kJ/mol��
| ||
�ʴ�Ϊ��CH4+H2O
| ||
��2��������ͼ1��֪���¶���ͬʱ����ѹǿΪP2ʱƽ��ʱCO��ת���ʸߣ��ɷ�ӦCO��g��+2H2��g��?CH3OH��g����֪ѹǿԽ��Խ������ƽ��������Ӧ���У���ѹǿP1��P2��
�ʴ�Ϊ������
���������������������£���Ӧ��3������a molCO��2amolH2����ЧΪ����ѹǿ��ƽ��������Ӧ�ƶ���COת�������ʴ�Ϊ������
��CO����ʼŨ��Ϊ
| a |
| V |
| 2a |
| V |
| a |
| V |
| a |
| 2V |
CO��g��+2H2��g��?CH3OH��g��
��ʼ��mol/L����
| a |
| V |
| 2a |
| V |
�仯��mol/L����
| a |
| 2V |
| a |
| V |
| a |
| 2V |
ƽ�⣨mol/L����
| a |
| 2V |
| a |
| V |
| a |
| 2V |
����ƽ�ⳣ��k=
| ||||
|
| V2 |
| a2 |
����ͬ�¶��£�CH3OH��g��?CO��g��+2H2��g����ƽ�ⳣ��Ϊ
| 1 | ||
|
| a2 |
| V2 |
�ʴ�Ϊ��
| a2 |
| V2 |
��3����Ӧ�����Ƕ����ѵ�ȼ�գ�ԭ��ظ�������������Ӧ���������ڸ����ŵ磬��ͼ��֪��a��Ϊ�����������ѷŵ����ɶ�����̼�������ӽ���Ĥ��֪�����������ӣ�a�缫�ĵ缫��ӦʽΪ��CH3OCH3-12e-+3H2O=2CO2+12H+���ʴ�Ϊ��CH3OCH3+3H2O-12e-=2CO2+12H+��
��4����֪����CO��g��+2H2��g��?CH3OH��g����H=-90.8kJ/mol��
��2CH3OH��g��?CH3OCH3��g��+H2O��g����H=-23.5kJ/mol��
��CO��g��+H2O��g��?CO2��g��+H2��g����H=-41.3kJ/mol��
�ɸ�˹���ɿ�֪���١�2+��+�۵�3CO��g��+3H2��g��?CH3OCH3��g��+CO2��g����H=-246.4kJ/mol��
�ʴ�Ϊ��-246.4kJ/mol��
���������⿼��Ӱ��ƽ������ء���ѧƽ�ⳣ������ѧƽ��ͼ��Ӧ�ȵļ���ȣ��Ѷ��еȣ���3����ע��������ӽ���Ĥ�ж��������������ǹؼ������������ܷ�Ӧʽ��ȥ������Ӧʽ������д��

��ϰ��ϵ�д�
�����Ŀ
һ���ɼ״���CH3OH���������Լ������������������Һ�����͵�أ��������ĵ缫��ӦʽΪ�� 2CH3 OH��16OH���D12e����2CO32����12H2O �� 6H2O��3O2��12e����12OH�������ڴ˵�ص��ƶ���ȷ���ǣ� ��
| A��ͨ��������һ���Ǹ��� |
| B���ŵ�ʱ����Һ�е�OH���������ƶ� |
| C����Ӧ������6mol���ӷ���ת�ƣ�����32g CH3 OH����ԭ |
| D���õ�ع���ʱ�״�һ��������Һ��pH���� |

