��Ŀ����
��ҵ�ϴӺ�ˮ����ȡ�����壬����һ�ֹ��ղ������·�����
(1)��ˮ��ͨ��Cl2������ˮ�е��廯�������������ӷ���ʽΪ��___________________��
(2)�������������Һ�д����ȿ����������ɵ��崵�����ô�����Һ���գ���ʱ�����ת���������Ӻ���������ӣ��仯ѧ����ʽΪ____________________________��
(3)��(2)������Һ��H2SO4�ữ���ֿɵõ������壬�����л��ܼ���ȡ����ɵõ�����ƷNa2SO4����һ���̿��û�ѧ����ʽ��ʾΪ��______________________��
(4)�����õ������л���������Cl2��������ȥ��д����Ӧ�����ӷ���ʽ��______________________________��
(1)��ˮ��ͨ��Cl2������ˮ�е��廯�������������ӷ���ʽΪ��___________________��
(2)�������������Һ�д����ȿ����������ɵ��崵�����ô�����Һ���գ���ʱ�����ת���������Ӻ���������ӣ��仯ѧ����ʽΪ____________________________��
(3)��(2)������Һ��H2SO4�ữ���ֿɵõ������壬�����л��ܼ���ȡ����ɵõ�����ƷNa2SO4����һ���̿��û�ѧ����ʽ��ʾΪ��______________________��
(4)�����õ������л���������Cl2��������ȥ��д����Ӧ�����ӷ���ʽ��______________________________��
(1)Cl2+2Br-=Br2+2Cl-
(2)3Na2CO3+3Br2=5NaBr+NaBrO3+3CO2��
(3)5NaBr+NaBrO3+3H2SO4=3Br2+3Na2SO4+3H2O
(4)Cl2+2Br-=Br2+2Cl-
(2)3Na2CO3+3Br2=5NaBr+NaBrO3+3CO2��
(3)5NaBr+NaBrO3+3H2SO4=3Br2+3Na2SO4+3H2O
(4)Cl2+2Br-=Br2+2Cl-

��ϰ��ϵ�д�
�����Ŀ
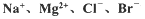 �ȣ���ģ�ҵ��������ȡþ����Ҫ�������£��ش��������⣺
�ȣ���ģ�ҵ��������ȡþ����Ҫ�������£��ش��������⣺