��Ŀ����
����(Na2S2O3��5H2O)���������ȼ�����Ӱ�����ⶾ������ۺ�����������Һ��п��Ƶ�Na2S2O3��5H2O��Na2S2O3��5H2O�IJ������ʼ��±���
ʵ�����Ʊ������������£�

(1)ʵ�鿪ʼʱ������1 mL��C2H5OH��Ŀ����________
(2)��ҺA�г���Na2S2O3������Na2SO3�⣬����ܴ��ڵ���������������________�������Һ�и����ʵĺ������ܵͣ�����ķ�����________
(3)���˲������õIJ���������________�����˺�������B������������ɫ���ʣ�Ϊ��ȥ����ɫ���ʣ�ϴ������B�IJ���������__________________
(4)�������BʱӦע��ʲô����________��ԭ����__________________
������
����(1)������ˮ����(2��)
����(2)Na2SO4(1��)��ȡ������Һ����ϡ�����ữ���ˣ��ټ�BaCl2��Һ(2��)
����(3)��������©�����ձ�(2��)�����Ҵ���CS2��û����������Ȼ���º��ظ�����(2��)��(��ˮϴ�Ӳ��÷�)
����(4)�¶�Ӧ����43��(1��)����ֹ����绯ʧˮ(1��)

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д����� (Na2S2O3•5H2O) ���������ȼ�����Ӱ�����ⶾ������ۺ�����������Һ��п��Ƶ� Na2S2O3•5H2O��Na2S2O3•5H2O�IJ������ʼ��±���
| �������� | ������ˮ���������Ҵ����۵� 48.2�棻�ڳ�ʪ�Ŀ������׳��� |
| ��ѧ���� | 43�����ϵĿ������绯�������ֽ� (S2O32+2H��=S��+SO2��+H2O ) |
ʵ�����Ʊ������������£�
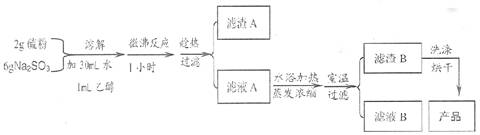
��1��ʵ�鿪ʼʱ������1 mL C2H5OH ��Ŀ����
��2����Һ A �г���Na2S2O3������ Na2SO3 �⣬����ܴ��ڵ���������������
�� �����Һ�и����ʵĺ������ܵͣ�����ķ�����
��3�����˲������õIJ��������� �� ���˺������� B ������������ɫ���ʡ�Ϊ��ȥ����ɫ���ʣ�ϴ������ B �IJ���������
��4��������� B ʱӦע��ʲô���� ��ԭ����
(11�� )���� (Na2S2O3•5H2O) ���������ȼ�����Ӱ�����ⶾ������ۺ�����������Һ��п��Ƶ� Na2S2O3•5H2O��Na2S2O3•5H2O�IJ������ʼ��±���
| �������� | ������ˮ���������Ҵ����۵� 48.2�棻�ڳ�ʪ�Ŀ������׳��� |
| ��ѧ���� | 43�����ϵĿ������绯�������ֽ� (S2O32�D+2H��=S��+SO2��+H2O ) |
ʵ�����Ʊ������������£�

��1��ʵ�鿪ʼʱ������1 mL C2H5OH ��Ŀ����
��2����Һ A �г���Na2S2O3������Na2SO3 �⣬����ܴ��ڵ��������������� �� �����Һ�и����ʵĺ������ܵͣ�����ķ�����
��3�����˲������õIJ��������� �� ���˺������� B ������������ɫ���ʡ�Ϊ��ȥ����ɫ���ʣ�ϴ������ B �IJ���������
��4��������� B ʱӦע��ʲô���� ��ԭ����
(11�� )���� (Na2S2O3•5H2O) ���������ȼ�����Ӱ�����ⶾ������ۺ�����������Һ��п��Ƶ� Na2S2O3•5H2O��Na2S2O3•5H2O�IJ������ʼ��±���
|
�������� |
������ˮ���������Ҵ����۵� 48.2�棻�ڳ�ʪ�Ŀ������׳��� |
|
��ѧ���� |
43�����ϵĿ������绯�������ֽ� (S2O32�D+2H��=S��+SO2��+H2O ) |
ʵ�����Ʊ������������£�

��1��ʵ�鿪ʼʱ������1 mL C2H5OH ��Ŀ����
��2����Һ A �г���Na2S2O3������ Na2SO3 �⣬����ܴ��ڵ��������������� �� �����Һ�и����ʵĺ������ܵͣ�����ķ�����
��3�����˲������õIJ��������� �� ���˺������� B ������������ɫ���ʡ�Ϊ��ȥ����ɫ���ʣ�ϴ������ B �IJ���������
��4��������� B ʱӦע��ʲô���� ��ԭ����