��Ŀ����
��֪A��B��C��D����ѧ��ѧ�г��������ֲ�ͬ��������֮���������ת����ϵ��
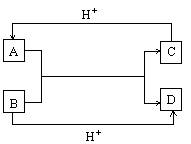
��1�����A��B��C��D����10���ӵ�������д����
A�Ľṹʽ________________________________��
D�ĵ���ʽ________________________________��
��2�����A��C��18���ӵ�����B��D��10���ӵ�������д����
��A��B����Һ�з�Ӧ�����ӷ���ʽ
________________________________________________��
�ڸ����������ӷ���ʽ�������ж�C��B������ӵ�������С�ǣ��û�ѧʽ�����ӷ��ű�ʾ��________________________>________________________��
�𰸣�
������
��ʾ��
������
��1��
��2����H2S+OH-�T�THS-+H2O��HS-+OH-�T�TS2-+H2O ��OH->S2-(HS-)
|
��ʾ��
10��������18���������ҷ����ֱ���Ne��ArΪ���գ��ֱ�����Ӧ��ԭ�ӣ��������Ӽ����ӡ���С��Χ�������ڿ��ٽ��⡣
|

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��֪A��B��C��D��ԭ��������������Ķ���������Ԫ�أ�A��B��C�ֱ��ڲ�ͬ���ڣ�A��Cͬ���壬B��һ�ֵ�����ʹ�����ǵ�ľ����ȼ��A��C��D����ԭ�ӵ�����������֮��Ϊ6������˵������ȷ���ǣ�������
| A��A��ԭ�Ӱ뾶��B��С | B��B��C�γɵĻ�����ֻ��һ�� | C��C�ڻ������г�+1�� | D��D���ʵľ���������뵼����� |
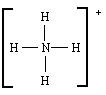 ��H��F��
��H��F��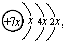 ��B��ͬ������ԭ�ӵ�һ��������С��Ԫ�أ�Cԭ�ӵ������������δ�ɶԵ��ӣ�E�������ճ���������õĽ�����
��B��ͬ������ԭ�ӵ�һ��������С��Ԫ�أ�Cԭ�ӵ������������δ�ɶԵ��ӣ�E�������ճ���������õĽ�����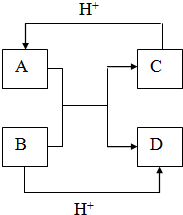 ��֪A��B��C��D����ѧ��ѧ�г��������ֲ�ͬ��������֮�������ͼ��ʾ��ת����ϵ��
��֪A��B��C��D����ѧ��ѧ�г��������ֲ�ͬ��������֮�������ͼ��ʾ��ת����ϵ��