��Ŀ����
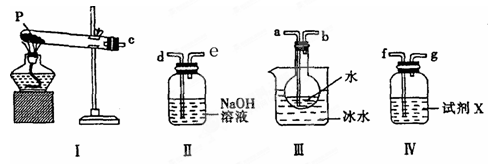
(10��)Ϊ�ⶨ�Ȼ��غ�����ػ�������Ȼ��صĺ�����ijͬѧ���������ʵ�飺������Ʒ���ܽ⣬��������A��Һ�����ˣ��ó�������ҺB��������ϴ�ӣ���ɣ�������C��
�ش��������⣺
��1����Һ�����ʵĻ�ѧʽ_____������C�Ļ�ѧʽ____��
��2����������ƽ������Ʒ������ƽƽ��ʱ���������̴�����������Ϊ26g������Ϊ0.4g����������Ʒʵ��������____��
��3��������ʹ�õ���������Ʒ���У���ֽ������̨����Ȧ���ձ���©��������Ҫ�������������Ʒ��______��
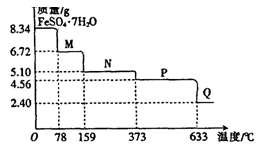
��4����C������Ϊ23.3g����ԭ��������Ȼ��ص���������Ϊ______��
���𰸡�
BaCl2��BaSO4��25.6g����������32%
����������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
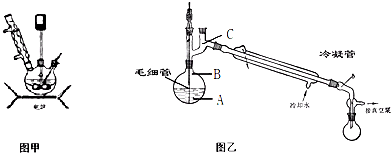
 ˮ������Ϊһ����Ҫ�ľ�ϸ����ԭ�ϣ���ũҩ��ҽҩ���л��ϳ����й㷺��;�� �����ط��Ʊ�ˮ���£��ɷ�Ϊ�����Σ���һ��Ϊ�����Ȼ��Σ��ڶ���Ϊ����ˮ��Σ��ܷ�Ӧ����ʽΪ����NH2��2CO+NaClO+2NaOH��H2N-NH2?H2O+NaCl+Na2CO3��
ˮ������Ϊһ����Ҫ�ľ�ϸ����ԭ�ϣ���ũҩ��ҽҩ���л��ϳ����й㷺��;�� �����ط��Ʊ�ˮ���£��ɷ�Ϊ�����Σ���һ��Ϊ�����Ȼ��Σ��ڶ���Ϊ����ˮ��Σ��ܷ�Ӧ����ʽΪ����NH2��2CO+NaClO+2NaOH��H2N-NH2?H2O+NaCl+Na2CO3��