��Ŀ����
���ͷ����ǻ���ԭ�ϡ����ڷ������ʻ��ã���������ȡ������
��1������ͨ����ⴿ����Һ̬HF���F2����ԭ����__________ �������Һ̬KHF2ʱ�������ֱ�õ�F2��H2��д��������HF2- �ŵ��������ĵ缫��Ӧʽ__________��
��2������K2MnF6��SbF5��һ�������·�����ӦҲ����ȡ����ͬʱ����KSbF6 ��MnF3����ѧ����ʽΪ____________�����л�ԭ������___________��
��3����֪�����������д�������ƽ�� ��H3F3 3HF ��H ��0, H2F2
3HF ��H ��0, H2F2  2HF ��H ��0��
2HF ��H ��0��
�����ڶ��¶����������ٳ���H3F3����H3F3��HF��Ũ�ȣ�mol��L����ֵ_________���������С�����䡱����ͬ����
�����ڶ��¶����������ٳ���HF����H3F3��HF��Ũ�ȣ�mol��L����ֵ__________��
��4��һ��Ũ�ȵ�HF��Al2(SO4)3���Һ�У����� ������������pH�ķֲ�������ͼʾ����NaOHʹ���Һ��pH��5������7��д���йط�Ӧ�����ӷ���ʽΪ___________ ��
��1������ͨ����ⴿ����Һ̬HF���F2����ԭ����__________ �������Һ̬KHF2ʱ�������ֱ�õ�F2��H2��д��������HF2- �ŵ��������ĵ缫��Ӧʽ__________��
��2������K2MnF6��SbF5��һ�������·�����ӦҲ����ȡ����ͬʱ����KSbF6 ��MnF3����ѧ����ʽΪ____________�����л�ԭ������___________��
��3����֪�����������д�������ƽ�� ��H3F3
 3HF ��H ��0, H2F2
3HF ��H ��0, H2F2  2HF ��H ��0��
2HF ��H ��0�������ڶ��¶����������ٳ���H3F3����H3F3��HF��Ũ�ȣ�mol��L����ֵ_________���������С�����䡱����ͬ����
�����ڶ��¶����������ٳ���HF����H3F3��HF��Ũ�ȣ�mol��L����ֵ__________��
��4��һ��Ũ�ȵ�HF��Al2(SO4)3���Һ�У����� ������������pH�ķֲ�������ͼʾ����NaOHʹ���Һ��pH��5������7��д���йط�Ӧ�����ӷ���ʽΪ___________ ��

��1���������ǹ��ۻ������Һ̬ʱ�����룬��������� �� HF2- -2e- =F2��+H+
��2��2K2MnF6 +4SbF5 =4KSbF6 + 2MnF3 +F2���� MnF3
��3������ �� ����
��4��AlF2��+3OH��=Al(OH)3��+2F����AlF3+3OH��=Al(OH)3��+3F��
��2��2K2MnF6 +4SbF5 =4KSbF6 + 2MnF3 +F2���� MnF3
��3������ �� ����
��4��AlF2��+3OH��=Al(OH)3��+2F����AlF3+3OH��=Al(OH)3��+3F��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��2011?����һģ�����ͷ����ǻ���ԭ�ϣ����ڷ������ʻ��ã���������ȡ������
��2011?����һģ�����ͷ����ǻ���ԭ�ϣ����ڷ������ʻ��ã���������ȡ������
 3HF ��H��0, H2F2
3HF ��H��0, H2F2
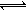 3HF ��H ��0, H2F2
3HF ��H ��0, H2F2 