��Ŀ����
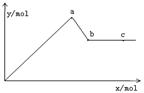
��֪Ba(AlO2)2������ˮ����ͼ��ʾ������A12(SO4)3 0.01mol����Һ����μ���Ba(OH)2��Һʱ�����ɳ��������ʵ���y�����Ba(OH)2�����ʵ���x�Ĺ�ϵ������a��c�ֱ���0b�κ�bd�ε��е㣩�������й�������ȷ����

A��aʱ����������Ϊ3.495 g
B��bʱ���������ʵ���Ϊ0.05 mol
C��cʱ��Һ��Ba2+���ӵ����ʵ���Ϊ0.005 mol
D��eʱ��Һ��AlO2�������ʵ���Ϊ0.01 mol
BC
��������
���������������������������Ӧ�ķ���ʽ��Al2(SO4)3��3Ba(OH)2��2Al(OH)3����3BaSO4�������������������������������������ʼ�ܽ⣬��Ӧ�Ļ�ѧ����ʽ��2Al(OH)3��Ba(OH)2��Ba(AlO2)2��4H2O������a��c�ֱ���0b�κ�bd�ε��е㣬����a������0.01mol����������0.015mol���ᱵ������֮����3.495g��0.78g��4.275g��A����ȷ��bʱ������0.02mol����������0.03mol���ᱵ�������ʵ���֮��Ϊ0.05 mol��B��ȷ��c����0.01mol���������ܽ⣬����0.005mol Ba(AlO2)2����ʱ��Һ��Ba2+���ӵ����ʵ���Ϊ0.005 mol��C��ȷ��e����Һ�еij���ֻ�����ᱵ������������ȫ�ܽ⣬����Һ��AlO2�������ʵ���Ϊ0.02mol��D����ȷ����ѡBC��
���㣺����������������������Ӧ���йؼ���
�����������Ǹ߿��еij������ͣ������ۺ�������Ŀ��飬���ض�ѧ�����������������������ۺ���ǿ�������߿���ּ������ѧ�����������ɡ��ܽ�����������������ڵ���ѧ����ѧϰ��Ȥ��ѧϰ�����ԣ�Ҳ����������ѧ���������������ͳ���˼ά����������Ĺؼ�����ȷ��Ӧԭ����Ȼ���������ͼ��������ü��ɡ�

��֪Ba(AlO2)2������ˮ������1 mol Al2(SO4)3����Һ�м��뺬��b mol Ba(OH)2 (b��6)����Һ�����ó��������ʵ���������Ϊ
| A��5mol | B��3mol | C��b/2mol | D��5b/3mol |
��֪Ba(AlO2)2������ˮ����ͼ��ʾ������A12(SO4)3 0.01mol����Һ����μ���Ba(OH)2��Һʱ�����ɳ��������ʵ���y�����Ba(OH)2�����ʵ���x�Ĺ�ϵ������a��c�ֱ���0b�κ�bd�ε��е㣩�������й�������ȷ����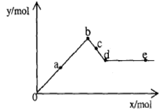
| A��aʱ����������Ϊ3.495 g |
| B��bʱ���������ʵ���Ϊ0.05 mol |
| C��cʱ��Һ��Ba2+���ӵ����ʵ���Ϊ0.005 mol |
| D��eʱ��Һ��AlO2�������ʵ���Ϊ0.01 mol |