��Ŀ����
7�����Ʊ�����TiO2�ķ���֮һ��TiCl4ˮ������TiO2•xH2O�������ˡ�ˮϴ��ȥ���е�Cl-���ٺ�ɡ����ճ�ȥˮ�ֵõ�����TiO2������NaOH�ⶨ��Һ��Ũ�ȣ�ȷ����1.000gNaOH�������������Ƴ�250mL��Һ��ȷ��ȡ25.00mL��Һװ����ƿ���μ�2�η�̪��ָʾ��������Һװ����ʽ�ζ����У�����Һ���ų����ݺ���Һ�İ�Һ��պ��ڡ�0���̶ȣ��ζ�NaOH��Һ���ﵽ�յ��¼������ʵ���ظ�3�Σ���¼���±���
| �ζ����� | NaOH��Һ���/mL | �ζ�������Һ���/mL |
| 1 | 25.00 | 20.02 |
| 2 | 25.00 | 17.10 |
| 3 | 25.00 | 19.98 |
��2�����Ƴ�250mL��Һʹ�õ�������250mL����ƿ��
��3���ζ��յ��������dz��ɫ��Һ��Ϊ��ɫ��������ڲ���ɫ��
��4����Һ�����ʵ����ʵ���Ũ��Ϊ0.125mol•L-1��
��5�����ڵζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ�棬ʹ�Բⶨ���ƫ�ߣ� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족��
���� ��1�����ݷ�Ӧ����������������غ㶨������д��ѧ����ʽ��
��2������������Һ�����ѡ������ƿ��
��3��������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��4���ȼ���NaOH��Ũ�ȣ����ڵڶ��εζ�ʱ�������������β��ϴ���ȥ����������Һ�����ƽ��ֵΪ$\frac{20.02+19.98}{2}$=20.00mL������c����Һ��=$\frac{c��NaOH����V��NaOH��}{V����Һ��}$���㣻
��5�����ӱ�ҺҺ�棬����Һ�����ƫС������c����Һ��=$\frac{c��NaOH����V��NaOH��}{V����Һ��}$������
��� �⣺��1��TiCl4ˮ������TiO2•xH2O����TiCl4��ϵ��Ϊ1������Ԫ���غ㣬TiO2•xH2O��ϵ��Ϊ1��HCl��ϵ��Ϊ4���ٸ���OԪ���غ㣬��֪H2O��ϵ��Ϊ��2+x����
�ʴ�Ϊ��TiCl4+��x+2��H2O?TiO2•xH2O+4HCl��
��2�����Ƴ�250mL��Һʹ�õ�������250mL����ƿ���ʴ�Ϊ��250mL����ƿ��
��3����̪������������Һ���Ժ�ɫ�����ŷ�Ӧ���м��Լ�������Һ��ɫ��dz������Һ��ɫ�ɷۺ�ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣬
�ʴ�Ϊ��dz��ɫ��Һ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
��4��1.000gNaOH�������������Ƴ�250mL��Һ����c��NaOH��=$\frac{n}{V}$=$\frac{\frac{1g}{40g/mol}}{0.25L}$=0.1mol/L��
�ڶ��εζ�ʱ�������������β��ϴ���ȥ����������Һ�����ƽ��ֵΪ$\frac{20.02+19.98}{2}$=20.00mL��
��c����Һ��=$\frac{c��NaOH����V��NaOH��}{V����Һ��}$=$\frac{0.1mol/L��0.025L}{0.02L}$=0.125 mol•L-1��
�ʴ�Ϊ��0.125 mol•L-1��
��5�����ڵζ��յ��ȡ�ζ��̶ܿ�ʱ������Һ�棬��ζ����ж�������Һ�����ƫС����c����Һ��=$\frac{c��NaOH����V��NaOH��}{V����Һ��}$��֪��������ƫ���ⶨ���ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
���� ���⿼������к͵ζ�����ȷ�к͵ζ�ʵ������������衢�յ���ж������ݴ����ȼ��ɽ��ע��ζ��ܵĶ���Ϊ�״��㣬��Ŀ�Ѷ��еȣ�

 ��������ϵ�д�
��������ϵ�д�| A�� | п����ϡH2SO4�ķ�Ӧ | B�� | Ba��OH��2•8H2O��NH4Cl�ķ�Ӧ | ||
| C�� | ���ȵ�̿��CO2�ķ�Ӧ | D�� | Ũ�����ϡ�� |
| A�� | ��ԭ�ӵ�ԭ�ӽṹʾ��ͼ��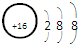 | |
| B�� | ԭ�Ӻ�����10�����ӵ���ԭ�ӣ�${\;}_{8}^{18}$O | |
| C�� | NH4Cl�ĵ���ʽ��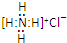 | |
| D�� | �����ᣨHClO4������Ԫ�صĻ��ϼ�Ϊ+5 |
| A�� | ���� | B�� | ���� | C�� | ��ը | D�� | ȼ�� |
| A�� | ��NaOH��Һ�ζ�����ʱ�����÷�̪��Һ��ָʾ�� | |
| B�� | �����͡����������͵�ˮ��ʱ�ɵõ�һ�ֹ�ͬ�IJ��� | |
| C�� | NH3+HCl=NH4Cl���Է����У����Hһ��С���� | |
| D�� | �����������Ӧ�뱽�����ᷴӦ�ķ�Ӧ���Ͳ�ͬ |
| A�� | ��Ԫ����������Һ������ɫ | |
| B�� | ������Ϊ86��������Ϊ51���ԭ�ӿɱ�ʾΪ��$\stackrel{137}{86}$Rn | |
| C�� | CS2�ĵ���ʽ����$\underset{\stackrel{..}{S}}{..}$��$\underset{\stackrel{..}{C}}{..}$��$\underset{\stackrel{..}{S}}{..}$�� | |
| D�� | 6.0g���ᾧ���к���H+����ĿΪ0.1NA |
| A�� | X��Y�γɵ����ֻ����������������ӵĸ����Ⱦ�Ϊ1��2 | |
| B�� | Y�ֱ���Z��W��R������Ԫ����ɵij�����������5�� | |
| C�� | Z��W��R����������Ӧˮ���������ǿ��˳���ǣ�R��W��Z | |
| D�� | Y���⻯���R���⻯���ȶ���Y���⻯���۷е��R���⻯��� |
| A�� | ��ɫ��ѧ�ĺ��������û�ѧԭ���Ի�����Ⱦ�������� | |
| B�� | ����ˮ����������ɱ��������˵�������� | |
| C�� | ���������������ͨ�������ճ�������Ҳ���ô�����Һ����ȥ��Ʒ��������� | |
| D�� | �Ӻ�ˮ����ȡ���ʶ�����ͨ����ѧ��Ӧ����ʵ�� |
