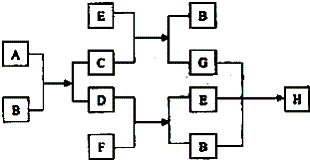
��Ŀ����
11����1���ڸ���ʱ��ˮ���������ȵ�̿����������ԭ��Ӧ�Ļ�ѧ����ʽ��H2O+C�TH2+CO���У�ˮ��������������������ȼ�յĻ�ѧ����ʽ��S+O2$\frac{\underline{\;��ȼ\;}}{\;}$SO2�����У����ǻ�ԭ������2��д��ʵ������ȡCO2�ķ�Ӧ�����ӷ���ʽ��CaCO3+2H+=CO2��+H2O+Ca2+
��3��д��Ba��NO3��2����Ļ�ѧ����ʽ��Ba��NO3��2=Ba2++2NO3-
��4����NAΪ�����ӵ�������ֵ�����agij�����к��еķ�����Ϊb����cg�������ڱ�״���µ������$\frac{22.4bc}{a{N}_{A}}$L��
���� ��1������Ԫ�ػ��ϼ۽��͵���������������������������ȼ�����ɶ�������
��2��̼����������ӷ�Ӧ���ɸ����ӡ�������̼��ˮ��
��3��ǿ���������Һ����ȫ���룻
��4������n=$\frac{N}{{N}_{A}}$����ag��������ʵ���������֮�ȵ��������ʵ���֮�ȣ��ݴ˼���cg����������ʵ���������V=n��Vm�����������
��� �⣺��1��H2O+C�TH2+CO��Ӧ�У�H2O��HԪ�ػ��ϼ۽��ͣ�ˮ����������������������ȼ�����ɶ��������䷴Ӧ����ʽΪ��S+O2$\frac{\underline{\;��ȼ\;}}{\;}$SO2����Ӧ��SԪ�صĻ��ϼ����ߣ�S����ԭ����
�ʴ�Ϊ��������S+O2$\frac{\underline{\;��ȼ\;}}{\;}$SO2����ԭ��
��2��̼����������ӷ�Ӧ���ɸ����ӡ�������̼��ˮ���䷴Ӧ�����ӷ���ʽΪ��CaCO3+2H+=CO2��+H2O+Ca2+��
�ʴ�Ϊ��CaCO3+2H+=CO2��+H2O+Ca2+��
��3��Ba��NO3��2����ǿ����ʣ�����Һ����ȫ���룬����뷽��ʽΪ��Ba��NO3��2=Ba2++2NO3-��
�ʴ�Ϊ��Ba��NO3��2=Ba2++2NO3-��
��4��a��ij�����к��еķ�����Ϊb����c�����庬�еķ�����Ϊ$\frac{cb}{a}$��c�˸���������ʵ���Ϊ$\frac{\frac{bc}{a}}{{N}_{A}}$=$\frac{bc}{{aN}_{A}}$mol���ڱ�״����Vm=22.4L/mol����cg����������Ϊ$\frac{bc}{{aN}_{A}}$mol��22.4L/mol=$\frac{22.4bc}{a{N}_{A}}$L��
�ʴ�Ϊ��$\frac{22.4bc}{a{N}_{A}}$L��
���� ���⿼����������ԭ��Ӧ�����ӷ�Ӧ�����ʵ����ļ��㣬������ѧ���ķ��������������Ŀ��飬ע��Թ�ʽ�����������Ӧ�ã��ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ������ĵ���ʽ��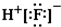 | B�� | HClO�Ľṹʽ��H-Cl-O | ||
| C�� | ���ĵ���ʽ 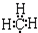 | D�� | ��̬��ԭ��L������Ų�ͼ��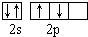 |
| A�� | 436kJ | B�� | 557kJ | C�� | 872kJ | D�� | 181kJ |
Ti��BH4��3��һ�ִ�����ϣ�����TiCl4��LiBH4��Ӧ�Ƶã�
��1����̬Ti3+��δ�ɶԵ�������1����
��2��LiBH4��Li+��BH4-���ɣ�LiBH4�в����ڵ���������C�����ţ���
A�����Ӽ� B�����ۼ� C�������� D����λ��
��3��Li��B��HԪ�صĵ縺���ɴ�С����˳��ΪH��B��Li��
��4������X����ͨ������γɡ���״�ṹ������ΪDZ�ڵĴ�����ϣ�Xһ������BC�����ţ���
A��H2O B��CH4 C��HF D��CO��NH2��2
��5���ء�þ�����γɵ�ij������ľ���ṹΪK+���������������ģ�Mg2+�ھ�����8�����ǣ�F-���ھ�����������ģ��ɼء�þ�����γɵĸû�����Ļ�ѧʽΪKMgF3��ÿ��K+��12��F-��λ��
��6���жϺ���������ǿ����һ����������Ǻ�������ӽṹ�к����ǻ���ԭ����Խ�࣬�ú����������Խǿ�����±���ʾ������������ǿ������ǻ���ԭ�����Ĺ�ϵ
| ������ | ���� | ���� | ������ | |
| ������ | Cl-OH |  |  |  |
| ���ǻ���ԭ���� | 0 | 1 | 2 | 3 |
| ���� | ���� | ��ǿ�� | ǿ�� | ��ǿ�� |
 ��H3PO3��H3AsO3�������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ�ֱ��ǣ�
��H3PO3��H3AsO3�������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ�ֱ��ǣ���H3PO3+2NaOH=Na2HPO3+2H2O��
��H3AsO3+3NaOH=Na3AsO3+3H2O��
 ��ͼ��ʾ����ƿ��ʢ������X���ι���ʢ��Һ��Y������ѹ�ιܽ�ͷ��ʹҺ��Y����ƿ�У�����һ����ɼ�С����a��������X��Һ��Y�������ǣ�������
��ͼ��ʾ����ƿ��ʢ������X���ι���ʢ��Һ��Y������ѹ�ιܽ�ͷ��ʹҺ��Y����ƿ�У�����һ����ɼ�С����a��������X��Һ��Y�������ǣ�������| A�� | X��NH3��Y��ˮ | B�� | X��CO2��Y�DZ���NaHCO3Ũ��Һ | ||
| C�� | X��SO2��Y��NaOH��Һ | D�� | X��HCl��Y��NaNO3ϡ��Һ |
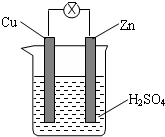
| A�� | �ձ�����Һ����ɫ | B�� | пƬ���ܽ� | ||
| C�� | ������ͭƬͨ����������пƬ | D�� | ��װ���ܹ�������ת��Ϊ��ѧ�� |
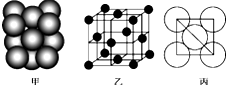 ͭ���ʾ�����ԭ�ӵĶѻ���ʽ��ͼ����ʾ���侧����ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ��ͼ����ʾ��
ͭ���ʾ�����ԭ�ӵĶѻ���ʽ��ͼ����ʾ���侧����ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ��ͼ����ʾ��