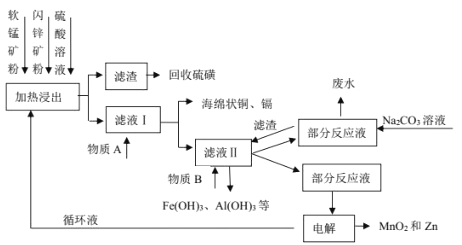
��Ŀ����
����Ŀ��������������(Na2S2O4)�׳Ʊ��շۣ���һ�ֵ���ɫ��ĩ��������ˮ���������Ҵ�����ʵ�����Ʊ��������������������£�
(1)��Ӧ�����Ʊ�SO2����ͼװ�ÿ���ȡ���������SO2��
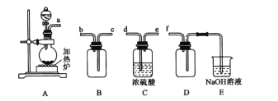
�ٰ������������Ӹ������ӿڣ�˳��Ϊa�� ___��f��װ��D��������______��
��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ___��
(2)��Ӧ������ʵ��װ����ͼ��ʾ(����װ��ʡ��)��
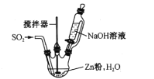
��ͨSO2֮ǰ��ǿ�����裬��п�ۺ�ˮ�Ƴ�����Һ����Ŀ����_________�����Ʒ�Ӧ�¶ȵķ�����____
�ڷ�Ӧ������ӷ���ʽΪ ___��
(3)����������ϴ�ӡ����գ��ɵõ�һ�ֹ�ҵ��Ʒ��____(�ѧʽ)��������������ʳ��ˮ��Ŀ���� ___��
(4)��ƷNa2S2O42H2O���ÿ������ױ����������������������_______(д2��)��
���𰸡�d��e��c��b ��ֹ���� Cu+2H2SO4(Ũ)![]() SO2��+CuSO4+2H2O �ӿ�SO2��Zn�ķ�Ӧ���� ˮԡ���� 2SO2+Zn+2OH-
SO2��+CuSO4+2H2O �ӿ�SO2��Zn�ķ�Ӧ���� ˮԡ���� 2SO2+Zn+2OH-![]() Zn(OH)2+ S2O42- ZnO ��СNa2S2O4���ܽ�� Na2SO3��NaHSO3��Na2SO4��NaHSO4(��д2��)
Zn(OH)2+ S2O42- ZnO ��СNa2S2O4���ܽ�� Na2SO3��NaHSO3��Na2SO4��NaHSO4(��д2��)
��������
(1)��ͭ��Ũ�����ڼ��������·�Ӧ��ȡ�����������ɵĶ��������к���ˮ��������Ҫ�������������ܶȱȿ���������ˮ��Ӧ��Ӧʹ�������ſ������ռ����ռ�ʱ����Ӧ�����̳������������ж��������ŷŵ���������Ҫβ������������β��ʱ����Ҫ��װ������װ�ã��������������Ӹ������ӿڣ�˳��Ϊa��d��e��c��b��f���������������������գ�ʹװ����ѹ��С��������������DΪ������װ�ã�
��ͭ��Ũ�����ڼ��������·�Ӧ���ɶ�����������ͭ��ˮ����ѧ����ʽΪ��Cu+2H2SO4(Ũ)![]() SO2��+CuSO4+2H2O��
SO2��+CuSO4+2H2O��
(2)��SO2��Zn��ֱ�ӽӴ���Ӧ���Ӵ����̫С��ͨSO2֮ǰ��ǿ�����裬��п�ۺ�ˮ�Ƴ�����Һ��������SO2��Zn�۷�Ӧʱ�ĽӴ�������Ӷ��ӿ�SO2��Zn�ķ�Ӧ���ʣ�������ͼʾ��Ӧ��ķ�Ӧ�¶�Ϊ35�棬�����¶�̫�߲����ƣ������þƾ���ֱ�Ӽ��ȣ�Ӧʹ��ˮԡ���ȵķ����������¶ȣ�
�ڶ���������п�ۺ��������Ʒ�Ӧ����������п��Na2S2O4�����ӷ���ʽΪ��2SO2+Zn+2OH-![]() Zn(OH)2+ S2O42-��
Zn(OH)2+ S2O42-��
(3)��(2)�ڿɵù��˵õ��ġ�������Ϊ������п��ϴ�ӡ����գ�������п�ֽ�Ϊ����п(ZnO)��Na2S2O4������ˮ����ȴ�ᾧ�����У�������������ʳ��ˮ���ɽ������ܽ�ȼӿ�ᾧ�ٶȣ�
(4)��ƷNa2S2O4��2H2O����Ļ��ϼ�Ϊ+3�ۣ�������ʱ��Ļ��ϼ����ߣ����ܱ�Ϊ+4�ۻ�+6�ۣ�����ܵIJ�����Na2SO3��NaHSO3��Na2SO4��NaHSO4(��д2��)��

 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д�����Ŀ���״�(CH3OH)������Ϊ��ɫҺ�壬��Ӧ�ù㷺�Ļ���ԭ�Ϻ�ǰ���ֹ۵�ȼ�ϡ�
(1)��֪��CH4(g)��H2O(g)CO(g)��3H2(g) H����206.0kJ/mol��1
CH4(g)��H2O(g)CH3OH(g)��H2(g) H����77.0kJ/mol��1
��CO��H2��Ӧ����CH3OH(g)���Ȼ�ѧ����ʽ��______________________��
(2)�״������ںϳ�3��5-�����������ӣ���Ӧ���£�
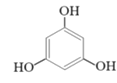 +2CH3OH
+2CH3OH![]()
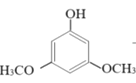 +2H2O
+2H2O
��Ӧ�������ȷ�����״����ټ������ѣ�����õ��л���(�������Ȼ���)����ϴ�ӣ�Ȼ������ᴿ�õ�����״���3��5-�����������ӵIJ����������ʼ�����
���� | �е�/�� | �۵�/�� | �ܽ��� |
�״� | 64.7 | ��97.8 | ������ˮ |
3��5-������������ | 172~175 | 33~36 | �����ڼ״������ѣ�����ˮ |
�ٷ�����״��IJ�����______________________(����ĸ���)��
a������ b����Һ c���ᾧ
��ϴ��ʱ�������ڳ�ȥ�л����е��Ȼ�����Լ���______________________(����ĸ���)��
a��Na2CO3��Һ b��NaHCO3��Һ c��NaOH��Һ
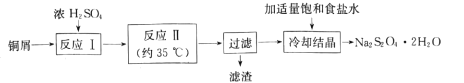
(3)�״�������ʵ�����Ʊ���Ȳ�����(CH![]() C��COOCH3���е�Ϊ103��105��)��
C��COOCH3���е�Ϊ103��105��)��
��ӦΪ��CH��C��COOH+CH3OH![]() CH��C��COOCH3+H2O
CH��C��COOCH3+H2O
ʵ�鲽�����£�
����1���ڷ�Ӧƿ�У�����14g��Ȳ�ᡢ50mL�״���2 mLŨ���ᣬ���裬���Ȼ���һ��ʱ�䡣
����2�����������ļ״�(װ����ͼ��ʾ)��

����3����ӦҺ��ȴ�������ñ���NaCl��Һ��5��Na2CO3��Һ��ˮϴ�ӡ�������л��ࡣ
����4���л��ྭ��ˮNa2SO4������ˡ����ñ�Ȳ�������
������A��������______________��������ƿ�м������Ƭ��Ŀ����_______________��
�ڲ���3�У���5%Na2CO3��Һϴ�ӣ���Ҫ��ȥ��������______________________��������л���IJ�������Ϊ_____________________��
�۲���4�У�����ʱ������ˮԡ���ȵ�ԭ����______________________��