��Ŀ����
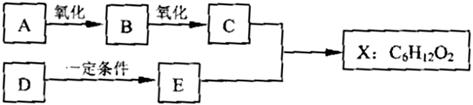
��֪X��һ�־��й���ζ�ĺϳ����ϣ���ͼΪ�ϳ�X��ij�����̣� E�������г�����һ���л��̼��������������ֱ�Ϊ52.17%��13.04%������Ϊ������������֪����Է�������Ϊ46���˴Ź���������ʾ����������ԭ�ӣ��Ҹ�����Ϊ1��2��3��

�����������Ϣ���ش��������⣺
��1��A�����й����ŵ������ǣ�
��2��D��E�Ļ�ѧ��Ӧ����Ϊ��
��3������A��B��C��D��E��X���������У���Ϊͬϵ����ǣ�
��4��C��һ��ͬ���칹��F���Է���ˮ�ⷴӦ��������Ӧ����F�Ľṹ��ʽΪ
��5����ӦC+E��X�Ļ�ѧ����ʽΪ

�����������Ϣ���ش��������⣺
��1��A�����й����ŵ������ǣ�
�ǻ�
�ǻ�
��E�Ľṹ��ʽ�ǣ�CH3CH2OH
CH3CH2OH
��2��D��E�Ļ�ѧ��Ӧ����Ϊ��
�ӳ�
�ӳ�
��Ӧ����3������A��B��C��D��E��X���������У���Ϊͬϵ����ǣ�
CH3CH2OH��CH3CH2CH2OH
CH3CH2OH��CH3CH2CH2OH
��4��C��һ��ͬ���칹��F���Է���ˮ�ⷴӦ��������Ӧ����F�Ľṹ��ʽΪ
HCOOC2H5
HCOOC2H5
����5����ӦC+E��X�Ļ�ѧ����ʽΪ
CH3CH2COOH+C2H5OH
CH3CH2COOC2H5+H2O
| Ũ���� |
| �� |
CH3CH2COOH+C2H5OH
CH3CH2COOC2H5+H2O
��| Ũ���� |
| �� |
������E�������г�����һ���л��̼��������������ֱ�Ϊ52.17%��13.04%������Ϊ������������֪����Է�������Ϊ46����N��C��=
=2��N��H��=
=6����N��O��=
=1��E�ķ���ʽΪC2H6O���˴Ź���������ʾ����������ԭ�ӣ��Ҹ�����Ϊ1��2��3����EΪCH3CH2OH�����ƿɵ�DΪCH2=CH2����X����ʽ��֪��CΪ���ᣬCΪCH3CH2COOH����A���������õ�C�����ƿɵ�BΪCH3CH2CHO��AΪCH3CH2CH2OH���ݴ˽��
| 46��52.17% |
| 12 |
| 46��13.04% |
| 1 |
| 46-12��2-6 |
| 16 |
����⣺E�������г�����һ���л��̼��������������ֱ�Ϊ52.17%��13.04%������Ϊ������������֪����Է�������Ϊ46����N��C��=
=2��N��H��=
=6����N��O��=
=1��E�ķ���ʽΪC2H6O���˴Ź���������ʾ����������ԭ�ӣ��Ҹ�����Ϊ1��2��3����EΪCH3CH2OH�����ƿɵ�DΪCH2=CH2����X����ʽ��֪��CΪ���ᣬCΪCH3CH2COOH����A���������õ�C�����ƿɵ�BΪCH3CH2CHO��AΪCH3CH2CH2OH��
��1��������������֪��AΪCH3CH2CH2OH�������ǻ���EΪCH3CH2OH���ʴ�Ϊ���ǻ���CH3CH2OH��
��2��D��E����ϩ���Ƿ����ӳɷ�Ӧ�����Ҵ����ʴ�Ϊ���ӳɷ�Ӧ��
��3������A��B��C��D��E��X���������У���Ϊͬϵ����ǣ�CH3CH2OH��CH3CH2CH2OH��
�ʴ�Ϊ��CH3CH2OH��CH3CH2CH2OH��
��4��CH3CH2COOH��һ��ͬ���칹��F���Է���ˮ�ⷴӦ��������Ӧ��FΪ�����γɵ�����Ϊ������������F�Ľṹ��ʽΪHCOOC2H5��
�ʴ�Ϊ��HCOOC2H5��
��5����ӦC+E��X�Ļ�ѧ����ʽΪ��CH3CH2COOH+C2H5OH
CH3CH2COOC2H5+H2O��
�ʴ�Ϊ��CH3CH2COOH+C2H5OH
CH3CH2COOC2H5+H2O��
| 46��52.17% |
| 12 |
| 46��13.04% |
| 1 |
| 46-12��2-6 |
| 16 |
��1��������������֪��AΪCH3CH2CH2OH�������ǻ���EΪCH3CH2OH���ʴ�Ϊ���ǻ���CH3CH2OH��
��2��D��E����ϩ���Ƿ����ӳɷ�Ӧ�����Ҵ����ʴ�Ϊ���ӳɷ�Ӧ��
��3������A��B��C��D��E��X���������У���Ϊͬϵ����ǣ�CH3CH2OH��CH3CH2CH2OH��
�ʴ�Ϊ��CH3CH2OH��CH3CH2CH2OH��
��4��CH3CH2COOH��һ��ͬ���칹��F���Է���ˮ�ⷴӦ��������Ӧ��FΪ�����γɵ�����Ϊ������������F�Ľṹ��ʽΪHCOOC2H5��
�ʴ�Ϊ��HCOOC2H5��
��5����ӦC+E��X�Ļ�ѧ����ʽΪ��CH3CH2COOH+C2H5OH
| Ũ���� |
| �� |
�ʴ�Ϊ��CH3CH2COOH+C2H5OH
| Ũ���� |
| �� |
���������⿼���л�����ƶϡ�ͬ���칹�塢�л���Ӧ���ͣ��漰ϩ��������ȩ�����ᡢ�������ʵȣ����ݼ���ȷ��E�Ľṹ�ǽ���ؼ���ע�����չ����ŵ�������ת����

��ϰ��ϵ�д�
�����Ŀ


 X�Ļ�ѧ��Ӧ������ ��Ӧ��
X�Ļ�ѧ��Ӧ������ ��Ӧ��
 X�Ļ�ѧ��Ӧ������ ��Ӧ��
X�Ļ�ѧ��Ӧ������ ��Ӧ��