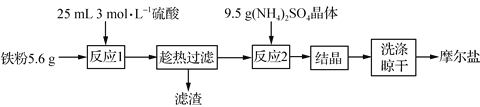
��Ŀ����
����Ŀ����NA��ʾ�����ӵ�������ֵ�������й�˵����ȷ����
A. 2.0gH182O��D2O�Ļ����������������ΪNA
B. ��״������22.4L���ȼ���ķ�����ԼΪNA��
C. 1molH2������O2��Ӧ���ɵ�H2O�к��еĹ��۽�����ΪNA
D. ��11P4+60CuSO4+96H2O=20Cu3P+24H3PO4+60H2SO4��Ӧ����6molCuSO4���������ķ�����Ϊ1.1NA
���𰸡�A
��������A��H218O��D2O��Ħ��������Ϊ20g/mol����2.0gH218O��D2O���������ʵ���Ϊ0.1mol����ÿ��H218O��D2O�����о���10�����ӣ���0.1 mol H218O��D2O������й���1mol���ӣ���NA������A��ȷ��B������£����ȼ���ΪҺ̬����22.4L���ȼ��鲻Ϊ1mol������������NA������B����C��1molH2������O2��Ӧ����1molH2O��ÿ��H2O���Ӻ���2��O-H����1molH2O�к��й��ۼ�����Ϊ2NA����C����D��CuSO4��CuԪ�ػ��ϼ���+2�۽���Cu3P �е�+1�ۣ���ÿ1molCuSO4��1mol���ӣ�6molCuSO4���Ե�6mol���ӣ�������P4����������Ϊ0�ۡ�+5�ۣ�ÿ1mol P4ʧȥ20mol���ӣ�����6molCuSO4������P4�����ʵ���Ϊ![]() mol����0.3mol����6molCuSO4���������ķ�����Ϊ0.3NA����D����ѡA��
mol����0.3mol����6molCuSO4���������ķ�����Ϊ0.3NA����D����ѡA��

����Ŀ�����ǻ�ѧʵ���Ҽ����������е���Ҫ���ʣ�Ӧ�ù㷺��
��1����֪25��ʱ��N2(g) �� O2(g) ![]() 2NO(g) ��H = +183kJ/mol
2NO(g) ��H = +183kJ/mol
2H2(g) �� O2(g) = 2H2O(l) ��H = ��571.6 kJ/mol
4NH3(g) �� 5O2(g) = 4NO(g) ��6H2O(l) ��H = ��1164.4 kJ/mol
�� N2(g) �� 3H2(g) ![]() 2NH3(g) ��H = kJ/mol
2NH3(g) ��H = kJ/mol
��2���ں��º�����ϵ�н��еĺϳɰ���Ӧ��������˵���÷�Ӧ�Ѵﵽƽ��״̬����___________
A.������N2��H2��NH3��Ũ��֮��Ϊ1�U3�U2
B.3v��N2������v��H2����
C.������ѹǿ���ֲ���
D.����1mol N2��ͬʱ����2molNH3
��3���ں��º����ܱ������н��кϳɰ���Ӧ����ʼͶ��ʱ������Ũ�����±���
N2 | H2 | NH3 | |
Ͷ�Ϣ� | 1.0 mol/L | 3.0 mol /L | 0 |
Ͷ�Ϣ� | 0.5 mol/L | 1.5 mol/L | 1.0 mol/L |
�ٰ�Ͷ�Ϣ���з�Ӧ����ôﵽ��ѧƽ��״̬ʱH2��ת����Ϊ40%������¶��ºϳɰ���Ӧ��ƽ�ⳣ��K=��ֻ��������ݣ����ü�������
�ڰ�Ͷ�Ϣ���з�Ӧ����ʼʱ��Ӧ���еķ���Ϊ�����������
��4���绯ѧ���������������ڼ�����NH3�ĺ������乤��ԭ��ʾ��ͼ���£�д���缫a�ĵ缫��Ӧʽ��