��Ŀ����
��һ������þ��ͭ��ɵĻ������뵽ϡHNO3�У�������ȫ�ܽ⣨���跴Ӧ�л�ԭ����ֻ��NO������Ӧ�����Һ�м���150mLһ��Ũ�ȵ�NaOH��Һǡ�ó�����ȫ��������ɳ�����������ԭ�Ͻ����������7.65g�������������в���ȷ����
- A.���õ�NaOH��Һ�����ʵ���Ũ��Ϊ3mol?L-1
- B.������ȫ���ܽ�ʱ���ռ���NO��������һ��Ϊ3.36L
- C.�μӷ�Ӧ�Ľ�������������m��Ϊ5.4g��m��14.4g
- D.��������������ȫ��ת��Ϊ���ᣬ��������Ҫ�����2.52L������
AB
�����������漰���ķ�Ӧ�У�3Mg+8HNO3 ��ϡ��=3Mg��NO3��2+2NO��+4H2O��3Cu+8HNO3 ��ϡ��=3Cu��NO3��2+2NO��+4H2O��
Mg��NO3��2+2NaOH=Mg��OH��2��+2NaNO3��Cu��NO3��2+2NaOH=Cu��OH��2��+2NaNO3��
��һ������þ��ͭ��ɵĻ������뵽ϡHNO3�У�������ȫ�ܽ⣨���跴Ӧ�л�ԭ����ֻ��NO������Ӧ�����Һ�м���15mLһ��Ũ�ȵ�NaOH��Һǡ�ó�����ȫ������Ϊ������þ��������ͭ�����ɳ�����������ԭ�Ͻ����������7.65g����������þ��������ͭ����������������Ϊ7.65g�������������ʵ���Ϊ =0.45mol����þ��ͭ���ܵ����ʵ���Ϊ0.225mol�����ݵ���ת���غ��֪���ɵ�NO���ʵ���Ϊ
=0.45mol����þ��ͭ���ܵ����ʵ���Ϊ0.225mol�����ݵ���ת���غ��֪���ɵ�NO���ʵ���Ϊ =0.15mol��
=0.15mol��
A��������������ƿ����к�Ϊ��Ӧ�����
B�����ݵ���ת���غ����NO���ʵ�����������岻һ���DZ�״���жϣ�
C��þ��ͭ���ܵ����ʵ���Ϊ0.225mol�����ü����ٶ�ȫΪþ��ȫΪͭ�����жϣ�
D������4NO+3O2+2H2O=4HNO3�����жϣ�
��𣺱����漰���ķ�Ӧ�У�3Mg+8HNO3 ��ϡ��=3Mg��NO3��2+2NO��+4H2O��3Cu+8HNO3 ��ϡ��=3Cu��NO3��2+2NO��+4H2O��
Mg��NO3��2+2NaOH=Mg��OH��2��+2NaNO3��Cu��NO3��2+2NaOH=Cu��OH��2��+2NaNO3��
��һ������þ��ͭ��ɵĻ������뵽ϡHNO3�У�������ȫ�ܽ⣨���跴Ӧ�л�ԭ����ֻ��NO������Ӧ�����Һ�м���15mLһ��Ũ�ȵ�NaOH��Һǡ�ó�����ȫ������Ϊ������þ��������ͭ�����ɳ�����������ԭ�Ͻ����������7.65g����������þ��������ͭ����������������Ϊ7.65g�������������ʵ���Ϊ =0.45mol����þ��ͭ���ܵ����ʵ���Ϊ0.225mol�����ݵ���ת���غ��֪���ɵ�NO���ʵ���Ϊ
=0.45mol����þ��ͭ���ܵ����ʵ���Ϊ0.225mol�����ݵ���ת���غ��֪���ɵ�NO���ʵ���Ϊ =0.15mol��
=0.15mol��
A����������ʣ�࣬���������Ƶ����ʵ�������0.45mol������������Һ��Ũ��Ϊ =3mol/L�����������ʣ�࣬��ʣ��������������Һ��Ũ�ȴ���3mol/L����A����
=3mol/L�����������ʣ�࣬��ʣ��������������Һ��Ũ�ȴ���3mol/L����A����
B��þ��ͭ���ܵ����ʵ���Ϊ0.225mol�����ݵ���ת���غ��֪���ɵ�NO���ʵ���Ϊ =0.15mol����Ϊ��״���£�����NO�����Ϊ0.15mol��22.4L/mol=3.36L����NO��һ�����ڱ�״������B����
=0.15mol����Ϊ��״���£�����NO�����Ϊ0.15mol��22.4L/mol=3.36L����NO��һ�����ڱ�״������B����
C��þ��ͭ���ܵ����ʵ���Ϊ0.225mol���ٶ�ȫΪþ������Ϊ0.225mol��24g/mol=5.4g����ȫΪͭ������Ϊ0.225mol��64g/mol=14.4g�����Բμӷ�Ӧ�Ľ�������������m��Ϊ5.4g��m��14.4g����C��ȷ��
D�����ݵ���ת���غ��֪���ɵ�NO���ʵ���Ϊ =0.15mol����4NO+3O2+2H2O=4HNO3��֪��������Ҫ���������ʵ���Ϊ0.15mol��
=0.15mol����4NO+3O2+2H2O=4HNO3��֪��������Ҫ���������ʵ���Ϊ0.15mol��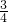 �����״�����������Ϊ0.15mol��
�����״�����������Ϊ0.15mol��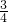 ��22.4L/mol=2.52L����D��ȷ��
��22.4L/mol=2.52L����D��ȷ��
��ѡAB��
������������þͭ�����ᷴӦ�����ɵ������������Ʒ�Ӧ���ۺϿ���ѧ���ĵ�ʧ�����غ㡢�����غ���ۺϼ���������ͬʱҲ������ѧ����ѧϰ�ۺ����úͽ�������������������һ�����������ĺ��⣬�ѶȽϴ�
�����������漰���ķ�Ӧ�У�3Mg+8HNO3 ��ϡ��=3Mg��NO3��2+2NO��+4H2O��3Cu+8HNO3 ��ϡ��=3Cu��NO3��2+2NO��+4H2O��
Mg��NO3��2+2NaOH=Mg��OH��2��+2NaNO3��Cu��NO3��2+2NaOH=Cu��OH��2��+2NaNO3��
��һ������þ��ͭ��ɵĻ������뵽ϡHNO3�У�������ȫ�ܽ⣨���跴Ӧ�л�ԭ����ֻ��NO������Ӧ�����Һ�м���15mLһ��Ũ�ȵ�NaOH��Һǡ�ó�����ȫ������Ϊ������þ��������ͭ�����ɳ�����������ԭ�Ͻ����������7.65g����������þ��������ͭ����������������Ϊ7.65g�������������ʵ���Ϊ
 =0.45mol����þ��ͭ���ܵ����ʵ���Ϊ0.225mol�����ݵ���ת���غ��֪���ɵ�NO���ʵ���Ϊ
=0.45mol����þ��ͭ���ܵ����ʵ���Ϊ0.225mol�����ݵ���ת���غ��֪���ɵ�NO���ʵ���Ϊ =0.15mol��
=0.15mol��A��������������ƿ����к�Ϊ��Ӧ�����
B�����ݵ���ת���غ����NO���ʵ�����������岻һ���DZ�״���жϣ�
C��þ��ͭ���ܵ����ʵ���Ϊ0.225mol�����ü����ٶ�ȫΪþ��ȫΪͭ�����жϣ�
D������4NO+3O2+2H2O=4HNO3�����жϣ�
��𣺱����漰���ķ�Ӧ�У�3Mg+8HNO3 ��ϡ��=3Mg��NO3��2+2NO��+4H2O��3Cu+8HNO3 ��ϡ��=3Cu��NO3��2+2NO��+4H2O��
Mg��NO3��2+2NaOH=Mg��OH��2��+2NaNO3��Cu��NO3��2+2NaOH=Cu��OH��2��+2NaNO3��
��һ������þ��ͭ��ɵĻ������뵽ϡHNO3�У�������ȫ�ܽ⣨���跴Ӧ�л�ԭ����ֻ��NO������Ӧ�����Һ�м���15mLһ��Ũ�ȵ�NaOH��Һǡ�ó�����ȫ������Ϊ������þ��������ͭ�����ɳ�����������ԭ�Ͻ����������7.65g����������þ��������ͭ����������������Ϊ7.65g�������������ʵ���Ϊ
 =0.45mol����þ��ͭ���ܵ����ʵ���Ϊ0.225mol�����ݵ���ת���غ��֪���ɵ�NO���ʵ���Ϊ
=0.45mol����þ��ͭ���ܵ����ʵ���Ϊ0.225mol�����ݵ���ת���غ��֪���ɵ�NO���ʵ���Ϊ =0.15mol��
=0.15mol��A����������ʣ�࣬���������Ƶ����ʵ�������0.45mol������������Һ��Ũ��Ϊ
 =3mol/L�����������ʣ�࣬��ʣ��������������Һ��Ũ�ȴ���3mol/L����A����
=3mol/L�����������ʣ�࣬��ʣ��������������Һ��Ũ�ȴ���3mol/L����A����B��þ��ͭ���ܵ����ʵ���Ϊ0.225mol�����ݵ���ת���غ��֪���ɵ�NO���ʵ���Ϊ
 =0.15mol����Ϊ��״���£�����NO�����Ϊ0.15mol��22.4L/mol=3.36L����NO��һ�����ڱ�״������B����
=0.15mol����Ϊ��״���£�����NO�����Ϊ0.15mol��22.4L/mol=3.36L����NO��һ�����ڱ�״������B����C��þ��ͭ���ܵ����ʵ���Ϊ0.225mol���ٶ�ȫΪþ������Ϊ0.225mol��24g/mol=5.4g����ȫΪͭ������Ϊ0.225mol��64g/mol=14.4g�����Բμӷ�Ӧ�Ľ�������������m��Ϊ5.4g��m��14.4g����C��ȷ��
D�����ݵ���ת���غ��֪���ɵ�NO���ʵ���Ϊ
 =0.15mol����4NO+3O2+2H2O=4HNO3��֪��������Ҫ���������ʵ���Ϊ0.15mol��
=0.15mol����4NO+3O2+2H2O=4HNO3��֪��������Ҫ���������ʵ���Ϊ0.15mol��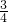 �����״�����������Ϊ0.15mol��
�����״�����������Ϊ0.15mol��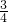 ��22.4L/mol=2.52L����D��ȷ��
��22.4L/mol=2.52L����D��ȷ����ѡAB��
������������þͭ�����ᷴӦ�����ɵ������������Ʒ�Ӧ���ۺϿ���ѧ���ĵ�ʧ�����غ㡢�����غ���ۺϼ���������ͬʱҲ������ѧ����ѧϰ�ۺ����úͽ�������������������һ�����������ĺ��⣬�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
��һ������þ��ͭ��ɵĻ������뵽ϡ�����У�������ȫ�ܽ⣨���跴Ӧ�л�ԭ����ֻ��NO������Ӧ�����Һ�м���3mol/L NaOH��Һ��������ȫ��������ɳ�����������ԭ�Ͻ����������5.1g�������������в���ȷ���ǣ�������
| A�������ɵij������ﵽ���ʱ������NaOH��Һ�����V��100mL | B��������ȫ���ܽ�ʱ�ռ���NO��������һ��Ϊ2.24L | C���μӷ�Ӧ�Ľ�����������Ϊ9.6g��m��3.6g | D��������ȫ���ܽ�ʱ���μӷ�Ӧ����������ʵ���һ����0.4mol |
��һ������þ��ͭ��ɵĻ������뵽ϡ�����У�������ȫ�ܽ⣨���跴Ӧ�л�ԭ����ֻ��NO������Ӧ�����Һ�м���3mol/L NaOH��Һ��������ȫ��������ɳ�����������ԭ�Ͻ����������5.1g�������������в���ȷ����
| A�������ɵij������ﵽ���ʱ������NaOH��Һ�����V��100mL |
| B��������ȫ���ܽ�ʱ�ռ���NO��������һ��Ϊ2.24L |
| C���μӷ�Ӧ�Ľ�����������Ϊ9.6g>m>3.6g |
| D��������ȫ���ܽ�ʱ���μӷ�Ӧ����������ʵ���һ����0.4mol |