��Ŀ����
��ľ���и������Σ���Ҫ�ɷ���̼��أ������ܺ�����������ء��Ȼ��ء��ִӲ�ľ������ȡ���Σ�����ʵ��������е� CO32����SO42���� Cl����
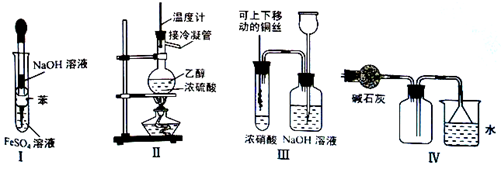
��1���Ӳ�ľ������ȡ���ε�ʵ�����˳�����£��ٳ�ȡ��Ʒ�����ܽ�������� ���� ������ȴ�ᾧ��
��2����������ƽ������Ʒʱ����ijͬѧ����Ʒ�������̣�����������̣�����ƽƽ��ʱ����Ϊ24.4g(һ������������) ������Ʒ��ʵ������Ϊ
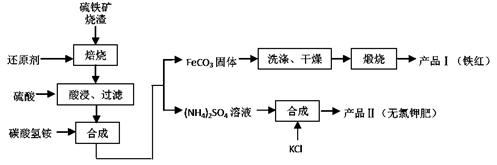
��3���ڽ��Тڡ��ۡ��� ����ʱ����Ҫ�õ��������������÷ֱ��ǣ�___________________��___________________��________________________��
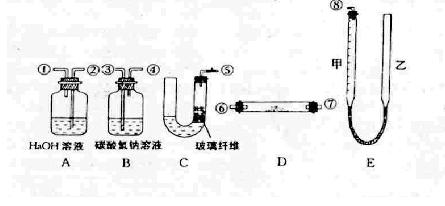
��4�����Ƶõ�������������Թܣ���������ˮ�ܽⲢ����Һ�ֳ����ݣ���װ��3֧�Թ��
���ڵ�һ֧�Թ������ϡ���ᣬ�ɹ۲쵽������Ϊ ��֤����Һ����CO32�����ӡ�
������ڵڶ�֧�Թ������SO42���Ĵ��� ��д���˹������漰��Ӧ�����ӷ���ʽ ��
���ڵ���֧�Թ�������������������۲쵽�г����������ɴ�֤����Һ��һ����Cl�����жϲ����ý����Ƿ����ܣ� ��
��1���Ӳ�ľ������ȡ���ε�ʵ�����˳�����£��ٳ�ȡ��Ʒ�����ܽ�������� ���� ������ȴ�ᾧ��
��2����������ƽ������Ʒʱ����ijͬѧ����Ʒ�������̣�����������̣�����ƽƽ��ʱ����Ϊ24.4g(һ������������) ������Ʒ��ʵ������Ϊ
��3���ڽ��Тڡ��ۡ��� ����ʱ����Ҫ�õ��������������÷ֱ��ǣ�___________________��___________________��________________________��
��4�����Ƶõ�������������Թܣ���������ˮ�ܽⲢ����Һ�ֳ����ݣ���װ��3֧�Թ��
���ڵ�һ֧�Թ������ϡ���ᣬ�ɹ۲쵽������Ϊ ��֤����Һ����CO32�����ӡ�
������ڵڶ�֧�Թ������SO42���Ĵ��� ��д���˹������漰��Ӧ�����ӷ���ʽ ��
���ڵ���֧�Թ�������������������۲쵽�г����������ɴ�֤����Һ��һ����Cl�����жϲ����ý����Ƿ����ܣ� ��
��1�����ˣ�ȡ��Һ ��1�֣� ������Һ��1�֣�
��2��23.6g ��1�֣�
��3�������ܽ⣨1�֣� ������1�֣� ���Ⱦ��ȣ���ֹҺ��ɽ�����1�֣�
��4���ٲ�����ɫ��ζ��ʹ����ʯ��ˮ����ǵ����壨д�����ݲ���Ҳ�ɵ÷֣���2�֣�
����ڶ�֧�Թ��м�������������ٵμ��Ȼ�����Һ���ɹ۲쵽�а�ɫ�������ɣ�֤����Һ����SO42�� ��2�֣�
2H+ + CO32���T�T CO2�� + H2O �� Ba2+ + SO42���T�T BaSO4�� ����2�֣���1�֣�
�۲����ܣ�̼����������Ҳ�������������ò��������� ��2�֣�
��2��23.6g ��1�֣�
��3�������ܽ⣨1�֣� ������1�֣� ���Ⱦ��ȣ���ֹҺ��ɽ�����1�֣�
��4���ٲ�����ɫ��ζ��ʹ����ʯ��ˮ����ǵ����壨д�����ݲ���Ҳ�ɵ÷֣���2�֣�
����ڶ�֧�Թ��м�������������ٵμ��Ȼ�����Һ���ɹ۲쵽�а�ɫ�������ɣ�֤����Һ����SO42�� ��2�֣�
2H+ + CO32���T�T CO2�� + H2O �� Ba2+ + SO42���T�T BaSO4�� ����2�֣���1�֣�
�۲����ܣ�̼����������Ҳ�������������ò��������� ��2�֣�
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
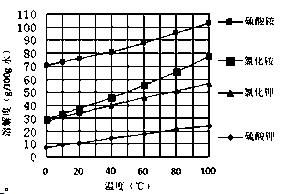
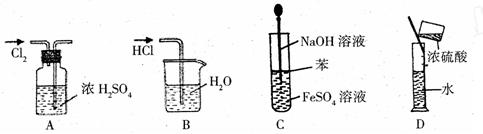
 2SO4��Һ�м���KCl��Һ����Ҫ���еIJ����ǣ���
2SO4��Һ�м���KCl��Һ����Ҫ���еIJ����ǣ��� OC�DCOOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������ijУ�о���ѧϰС��Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺
OC�DCOOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������ijУ�о���ѧϰС��Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺 HCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ_______
HCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ_______ __________________________________��
__________________________________��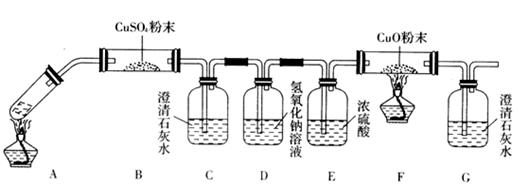
 ��Һ��ȥ
��Һ��ȥ ���Ȼ������ɫ����
���Ȼ������ɫ����