��Ŀ����
������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㡣
��֪��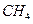 ��g��+
��g��+ 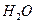 ��g��====
��g��====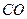 ��g��+
��g��+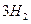 ��g��
��g��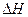 =
= 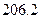
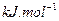
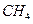 ��g��+
��g��+ 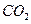 ��g��====
��g��==== ��g��+
��g��+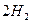 ��g��
��g��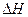 =
= 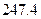
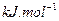
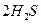 ��g��====
��g��====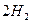 ��g��+
��g��+ ��g��
��g�� 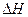 =
= 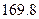
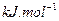
��1���Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ����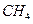 ��g����
��g����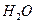 ��g����Ӧ����
��g����Ӧ����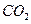 ��g����
��g����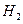 ��g�����Ȼ�ѧ����ʽΪ______��
��g�����Ȼ�ѧ����ʽΪ______��
��2��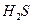 �ȷֽ�����ʱ������Ӧ����ͨ��һ������������ʹ����
�ȷֽ�����ʱ������Ӧ����ͨ��һ������������ʹ����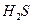 ȼ�գ���Ŀ����_____;ȼ�����ɵ�
ȼ�գ���Ŀ����_____;ȼ�����ɵ�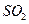 ��
��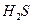 ��һ����Ӧ���������ڳ����¾������壬д���÷�Ӧ�Ļ�ѧ����ʽ��_______��
��һ����Ӧ���������ڳ����¾������壬д���÷�Ӧ�Ļ�ѧ����ʽ��_______��
��3��H O���ȷֽ�Ҳ�ɵõ�H
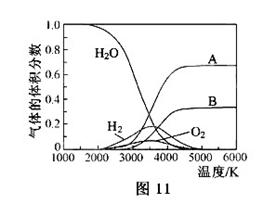
O���ȷֽ�Ҳ�ɵõ�H ��������ˮ�ֽ���ϵ����Ҫ���������������¶ȵĹ�ϵ��ͼ11��ʾ��ͼ��A��B��ʾ������������_______��
��������ˮ�ֽ���ϵ����Ҫ���������������¶ȵĹ�ϵ��ͼ11��ʾ��ͼ��A��B��ʾ������������_______��
��4���������[CO(NH )
)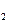 ]�ļ�����Һ�����װ��ʾ��ͼ��ͼ12�������и�Ĥ����ֹ����ͨ��������������Ϊ���Ե缫�������ʱ�������ĵ缫��ӦʽΪ_______��
]�ļ�����Һ�����װ��ʾ��ͼ��ͼ12�������и�Ĥ����ֹ����ͨ��������������Ϊ���Ե缫�������ʱ�������ĵ缫��ӦʽΪ_______��
��5��Mg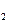 Cu��һ�ִ���Ͻ�350��ʱ��Mg
Cu��һ�ִ���Ͻ�350��ʱ��Mg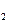 Cu��H
Cu��H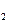 ��Ӧ������MgCu
��Ӧ������MgCu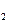 �ͽ���һ�ֽ���Ԫ�ص��⻯����������������Ϊ0.077����Mg
�ͽ���һ�ֽ���Ԫ�ص��⻯����������������Ϊ0.077����Mg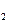 Cu��H
Cu��H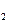 ��Ӧ�Ļ�ѧ����ʽΪ_______��
��Ӧ�Ļ�ѧ����ʽΪ_______��
��֪��
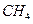 ��g��+
��g��+ 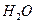 ��g��====
��g��====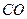 ��g��+
��g��+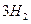 ��g��
��g��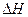 =
= 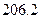
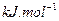
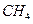 ��g��+
��g��+ 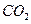 ��g��====
��g��==== ��g��+
��g��+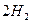 ��g��
��g��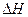 =
= 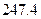
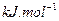
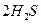 ��g��====
��g��====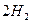 ��g��+
��g��+ ��g��
��g�� 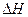 =
= 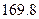
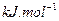
��1���Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ����
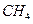 ��g����
��g����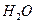 ��g����Ӧ����
��g����Ӧ����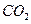 ��g����
��g����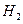 ��g�����Ȼ�ѧ����ʽΪ______��
��g�����Ȼ�ѧ����ʽΪ______����2��
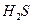 �ȷֽ�����ʱ������Ӧ����ͨ��һ������������ʹ����
�ȷֽ�����ʱ������Ӧ����ͨ��һ������������ʹ����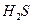 ȼ�գ���Ŀ����_____;ȼ�����ɵ�
ȼ�գ���Ŀ����_____;ȼ�����ɵ�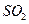 ��
��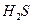 ��һ����Ӧ���������ڳ����¾������壬д���÷�Ӧ�Ļ�ѧ����ʽ��_______��
��һ����Ӧ���������ڳ����¾������壬д���÷�Ӧ�Ļ�ѧ����ʽ��_______����3��H
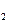 O���ȷֽ�Ҳ�ɵõ�H
O���ȷֽ�Ҳ�ɵõ�H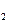 ��������ˮ�ֽ���ϵ����Ҫ���������������¶ȵĹ�ϵ��ͼ11��ʾ��ͼ��A��B��ʾ������������_______��
��������ˮ�ֽ���ϵ����Ҫ���������������¶ȵĹ�ϵ��ͼ11��ʾ��ͼ��A��B��ʾ������������_______��
��4���������[CO(NH
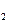 )
)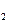 ]�ļ�����Һ�����װ��ʾ��ͼ��ͼ12�������и�Ĥ����ֹ����ͨ��������������Ϊ���Ե缫�������ʱ�������ĵ缫��ӦʽΪ_______��
]�ļ�����Һ�����װ��ʾ��ͼ��ͼ12�������и�Ĥ����ֹ����ͨ��������������Ϊ���Ե缫�������ʱ�������ĵ缫��ӦʽΪ_______��
��5��Mg
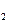 Cu��һ�ִ���Ͻ�350��ʱ��Mg
Cu��һ�ִ���Ͻ�350��ʱ��Mg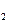 Cu��H
Cu��H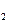 ��Ӧ������MgCu
��Ӧ������MgCu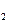 �ͽ���һ�ֽ���Ԫ�ص��⻯����������������Ϊ0.077����Mg
�ͽ���һ�ֽ���Ԫ�ص��⻯����������������Ϊ0.077����Mg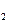 Cu��H
Cu��H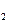 ��Ӧ�Ļ�ѧ����ʽΪ_______��
��Ӧ�Ļ�ѧ����ʽΪ_______����1��CH4(g)+2H2O(g) ="==" CO2(g)+4H2(g) 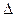 H =" 165.0" kJ��mol-1
H =" 165.0" kJ��mol-1
��2�� ΪH2S�ȷֽⷴӦ�ṩ����
2H2S+SO2 ="==" 2H2O+3S (��4H2S+2SO2 ="==" 4H2O+3S2)
��3��H��O������ԭ�ӡ���ԭ�ӣ�

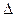 H =" 165.0" kJ��mol-1
H =" 165.0" kJ��mol-1��2�� ΪH2S�ȷֽⷴӦ�ṩ����
2H2S+SO2 ="==" 2H2O+3S (��4H2S+2SO2 ="==" 4H2O+3S2)
��3��H��O������ԭ�ӡ���ԭ�ӣ�

��

��ϰ��ϵ�д�
 �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
�����Ŀ
 O 2��g��==H2O��g�� ��H="a" kJ��mol-1��
O 2��g��==H2O��g�� ��H="a" kJ��mol-1�� CO2 (g)+ H2 (g)��Ӧ���������仯����ͼ��ʾ���÷�ӦΪ ��Ӧ������ȡ����ȡ�������Ӧ���Ȼ�ѧ����ʽΪ�� ��
CO2 (g)+ H2 (g)��Ӧ���������仯����ͼ��ʾ���÷�ӦΪ ��Ӧ������ȡ����ȡ�������Ӧ���Ȼ�ѧ����ʽΪ�� ��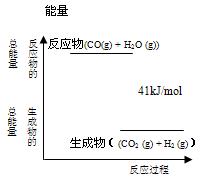
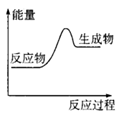
 Cu2O + H2����
Cu2O + H2����
 ��N2�����Ʒ��Ļ�ѧ����ʽΪ �� ��
��N2�����Ʒ��Ļ�ѧ����ʽΪ �� �� ��H>0��ˮ������Ũ����ʱ��t�仯���±���ʾ��
��H>0��ˮ������Ũ����ʱ��t�仯���±���ʾ��