��Ŀ����
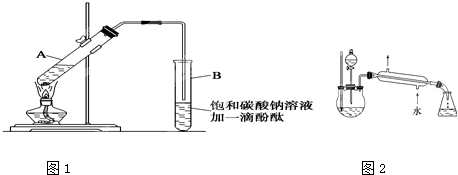
��֪�Ҵ����Ժ��Ȼ��Ʒ�Ӧ��������ˮ��CaCl2��6C2H5OH���йص��л��Լ��ķе����£�CH3COOC2H5��77.1�棻C2H5OH��78.3�棻C2H5OC2H5�����ѣ���34.5�棻CH3COOH��118�档ʵ���Һϳ����������ֲ�Ʒ�IJ������£�
������������ƿ�ڽ��������Ҵ���������Ũ�����ϣ�Ȼ��Һ©���ߵμӴ��ᣬ�������õ������Ҵ������ѡ������ˮ�����������ֲ�Ʒ����ش��������⣺
��1����Ӧ�м�����Ҵ��ǹ����ģ���Ŀ����______________________��
��2���ߵμӴ��ᣬ���������Ŀ����___________________________��
���ֲ�Ʒ�پ����в��辫�ƣ�
��3����ȥ���еĴ��ᣬ�����Ʒ�м��루����ĸ��_________________��
A����ˮ�Ҵ��������������������� B��̼���Ʒ�ĩ�������������������� C����ˮ������
��4�������м��뱥���Ȼ�����Һ�������롣��Ŀ����_____________��
��5��Ȼ���������м�����ˮ�����ƣ�����Ŀ����_________________��
����������������������Һ�������һ�������ƿ�ڣ���������ȥ�ͷе���֣��ռ��г�76��78��֮�����ּ�������������
������
��1������Ӧ���Ҵ���Ũ�ȣ������ڷ�Ӧ���������������ķ�����У�2�����Ӵ���Ũ�ȣ���С����������������Ũ��������������Ӧ���������������ķ������ ��3��B ��4����ȥ�ֲ�Ʒ�е��Ҵ� ��5����ȥ�ֲ�Ʒ�е�ˮ
|

 CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O