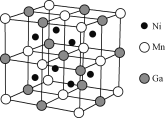
��Ŀ����
����Ŀ����֪��һԪ����HA�ĵ���ƽ�ⳣ��K ��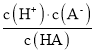 ��25��ʱ��CH3COOH��HCN��H2CO3�ĵ���ƽ�ⳣ�����£�
��25��ʱ��CH3COOH��HCN��H2CO3�ĵ���ƽ�ⳣ�����£�
��ѧʽ | CH3COOH | HCN | H2CO3 |
K | 1.75��10�C5 | 4.9��10�C10 | K1 = 4.4��10�C7 K2 = 5.6��10�C11 |
����˵����ȷ����
A. ϡ��CH3COOH��Һ�Ĺ����У�n(CH3COO�C)��С
B. NaHCO3��Һ�У�c(H2CO3) �� c(![]() ) �� c(HCO3)
) �� c(HCO3)
C. 25��ʱ����ͬ���ʵ���Ũ�ȵ�NaCN��Һ�ļ���ǿ��CH3COONa��Һ
D. ��CH3COOH��Һ��HCN��Һ�м���Na2CO3��Һ��������CO2
���𰸡�C
��������
A����ˮϡ��CH3COOH��Һ���ٽ�CH3COOH�ĵ��룬��Һ��n(CH3COO�C)�����࣬��A����B��NaHCO3��Һ��HCO3-��ˮ�ⳣ��Kh2=![]() =
=![]() =2.27��10�C8��K2 = 5.6��10�C11��˵��HCO3-��ˮ����ڵ��룬����Һ��c(
=2.27��10�C8��K2 = 5.6��10�C11��˵��HCO3-��ˮ����ڵ��룬����Һ��c(![]() )��c(H2CO3)�� c(HCO3)����B����C���ɵ���ƽ�ⳣ����֪Ũ�����ʱHCN������С��CH3COOH��ͬCN-��ˮ��̶ȴ���CH3COO-����25��ʱ����ͬ���ʵ���Ũ�ȵ�NaCN��Һ�ļ���ǿ��CH3COONa��Һ����C��ȷ��D���ɵ���ƽ�ⳣ����֪Ũ�����ʱHCN������С��H2CO3������HCN��Һ�м���Na2CO3��Һ��ֻ������NaHCO3����CO2�������ɣ���D���ʴ�ΪC��
)��c(H2CO3)�� c(HCO3)����B����C���ɵ���ƽ�ⳣ����֪Ũ�����ʱHCN������С��CH3COOH��ͬCN-��ˮ��̶ȴ���CH3COO-����25��ʱ����ͬ���ʵ���Ũ�ȵ�NaCN��Һ�ļ���ǿ��CH3COONa��Һ����C��ȷ��D���ɵ���ƽ�ⳣ����֪Ũ�����ʱHCN������С��H2CO3������HCN��Һ�м���Na2CO3��Һ��ֻ������NaHCO3����CO2�������ɣ���D���ʴ�ΪC��

 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д� �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�����Ŀ��1��2-���ȱ���(CH2C1CHClCH3)��һ����Ҫ�Ļ���ԭ�ϣ���ҵ�Ͽ��ñ�ϩ�ӳɷ��Ʊ�����Ҫ������Ϊ3-�ȱ�ϩ(CH2=CHCH2C1)����Ӧԭ��Ϊ��
I��CH2=CHCH3(g)+C12(g)![]() CH2C1CHC1CH3(g) ��H1=��134kJ��mol-1
CH2C1CHC1CH3(g) ��H1=��134kJ��mol-1
II��CH2=CHCH3(g)+C12(g)![]() CH2=CHCH2C1(g)+HC1(g)��H2=��102kJ��mol-1
CH2=CHCH2C1(g)+HC1(g)��H2=��102kJ��mol-1
��ش��������⣺
(1)��֪CH2=CHCH2C1(g)+HC1(g)![]() CH2C1CHC1CH3(g)�Ļ��Ea(��)Ϊ132kJ��mol-1����÷�Ӧ�Ļ��Ea(��)Ϊ______kJ��mol-1��
CH2C1CHC1CH3(g)�Ļ��Ea(��)Ϊ132kJ��mol-1����÷�Ӧ�Ļ��Ea(��)Ϊ______kJ��mol-1��
(2)һ���¶��£�������ܱ������г�������ʵ�����CH2=CHCH3(g)��C12(g)���ڴ��������·�����ӦI���������������ѹǿ��ʱ��ı仯���±���ʾ��
ʱ��/min | 0 | 60 | 120 | 180 | 240 | 300 | 360 |
ѹǿ/kPa | 80 | 74.2 | 69.4 | 65.2 | 61.6 | 57.6 | 57.6 |
���õ�λʱ���������ѹ�ı仯����ʾ��Ӧ���ʣ���![]() ����ǰ120min��ƽ����Ӧ����v(CH2C1CHC1CH3)=______kPa��min-1��(����С�����2λ)��
����ǰ120min��ƽ����Ӧ����v(CH2C1CHC1CH3)=______kPa��min-1��(����С�����2λ)��
�ڸ��¶��£���ƽ��ʱHC1���������Ϊ![]() �����ϩ��ƽ����ת����
�����ϩ��ƽ����ת����![]() _______����ӦI��ƽ�ⳣ��Kp=_____kPa-1(KpΪ�Է�ѹ��ʾ��ƽ�ⳣ��������С�����2λ)��
_______����ӦI��ƽ�ⳣ��Kp=_____kPa-1(KpΪ�Է�ѹ��ʾ��ƽ�ⳣ��������С�����2λ)��
(3)ij�о�С�����ܱ������г���һ������CH2=CHCH3��C12���ֱ���A��B���ֲ�ͬ���������·�����Ӧ��һ��ʱ�����CH2C1CHC1CH3�IJ������¶ȵĹ�ϵ����ͼ��ʾ��
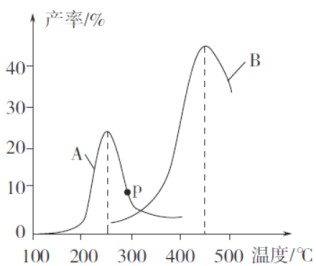
������˵���������___________(�����)��
a��ʹ�ô���A������¶�ԼΪ250��
b����ͬ�����£��ı�ѹǿ��Ӱ��CH2C1CHC1CH3�IJ���
c�����ִ������ܽ��ͷ�Ӧ�Ļ�ܣ�����H����
d�����CH2C1CHC1CH3��Ӧѡ���ԵĹؼ������ǿ����¶�
���ڴ���A�����£��¶ȵ���200��ʱ��CH2C1CHC1CH3�IJ������¶����߱仯������Ҫԭ����_______________________________________________________________��
��p���Ƿ�Ϊ��Ӧ�¶���CH2C1CHC1CH3��ƽ����ʣ��ж�������_____________��
����Ŀ��ijС��ͬѧ�о�SO2��KI��Һ�ķ�Ӧ���������ʵ�顣
ʵ�� | ���� | ���� |
I |
| ��ҺѸ�ٱ�Ϊdz��ɫ������Һ�������ķ������������ ���������Һ������ɫ |
II |
| ��Һ����������ɫ������Һ�������ķ�����dz��ɫ�����������Һ��ɫ��dz�����������Һ������ɫ |
��1�����������Һ��Ŀ����______��Ϊ�ﵽ��ͬ��Ŀ�ģ�����ѡ�õ��Լ���______��
��2�������飬II�е�dz��ɫ��������������ʾ�������������£�SO2��KI��Һ��Ӧ����S��I2��
�� �����������£�SO2��KI��Һ��Ӧ�����ӷ���ʽ��______��
�� ���II�м��������Һ����ɫ����ͬѧ������裺______��Ϊ֤ʵ�ü��裬��ͬѧȡII�����ķ�������Һ�����������ữ��BaCl2��Һ���а�ɫ�������ɡ�
�� ��ͬѧ��Ϊ��ͬѧ��ʵ�鷽����֧���������裬������______��
�� ��ͬѧ��1 mL 1 mol��L1 KCl��Һ�м���5��1 mol��L1���ᣬͨ��SO2��������ʵ�������ټ��������ữ��BaCl2��Һ������û�а�ɫ��������ͬѧ��ʵ�������______��
��3��ʵ�������I��SO2ת���Ĵ�������ȫ�������ӷ���ʽ��
SO2��H2O ===_____ �� ��
��4���Ա�ʵ��I��II���Ʋ�����H+��Ũ�ȿɼӿ�SO2��ת�����ʡ�Ϊ֤ʵ���Ʋ⣬�����һ������ʵ��֤����ʵ�鷽����______��