��Ŀ����
����Ŀ���ڱ�״���½��мס��ҡ�������ʵ�飺�����ȡ30.0 mLͬŨ�ȵ�������Һ������ͬһ��þ���Ͻ��ĩ���������壬�й������б����£�
ʵ����� | �� | �� | �� |
�Ͻ�����/mg | 255 | 385 | 459 |
�����������/mL | 280 | 336 | 336 |
��ش�
(1)����ʵ���У�����______________��ѡ���������������������������ͬ����������______________��Ҫ�����������ʵ���Ũ�ȣ����п����������ݵ�������______________����õ���������ʵ���Ũ��Ϊ______________��
(2)��Ͻ���Mg��Al�����ʵ���֮�ȣ����п����������ݵ�������______________����õ�Mg��Al�����ʵ���֮��Ϊ______________��
(3)�ڱ���ʵ��֮���������м���1.00 mol��L��1 NaOH��Һ����ʹ�Ͻ��е���ǡ���ܽ⣬���γ����ij�������ʹMg2���պó�����ȫ���ٹ��˳������Թ��壬����Һ�и����ʵ����ʵ�����������NaOH��Һ�������д������̣���___________________
���𰸡����� ͬ����������ʱ����H2���� 336mL�����30mL��Һ 1mol/L 255mg��280mL 1��1 NaCl 0.03mol NaAlO2 0.009mol 39 mL
��������
(1)����ʵ��֪�����ӺϽ�������������������˵����ʵ�����������ʣ�ࡣ��Ϊ������ͬ����������ʱ����H2���١�
�Ƚ��Һͱ�ʵ�飬����������ͬ��˵����ʵ�������Ѿ���ȫ��Ӧ������336mL�����30mL��Һ�Ǽ������Ũ������ʹ�õ����ݡ���Ϊ336mL�����30mL��Һ��
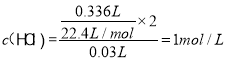 ��Ϊ1mol/L��
��Ϊ1mol/L��
(2)�Ƚϼס���ʵ�����ݿ�֪���������������Ͻ���ȫ��Ӧ������255mg��280mL�Ǽ���Ͻ���Mg��Al�����ʵ���֮������ʹ�õ����ݡ���Ϊ255mg��280mL��
��Mg�����ʵ���Ϊx��Al�����ʵ���Ϊy���з�����Ϊ��
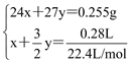 �����x��y��1��1����Ϊ1��1��
�����x��y��1��1����Ϊ1��1��
(3)����Cl-�غ㣬�ɵã�n(NaCl)��n(HCl)��1mol/L��0.03L��0.03mol��
����Al�غ㣬�ɵã�n(NaAlO2)��n(Al)�� ��0.009 mol��
��0.009 mol��
����Na���غ㣬�ɵã�n(NaOH)��0.03mol��0.009mol��0.039mol������V(NaOH)��39 mL��
����NaCl 0.03mol NaAlO2 0.009mol V(NaOH)��39 mL��