��Ŀ����
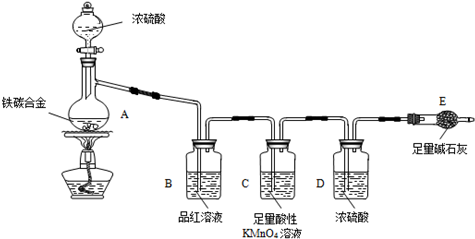
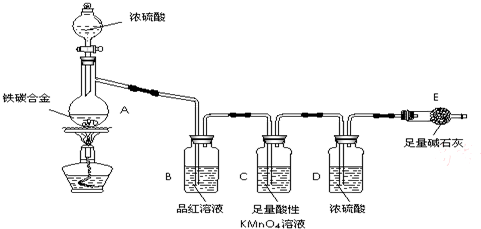
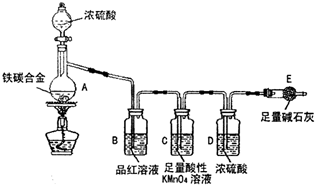 ij��ѧ��ȤС��Ϊ�˲ⶨij��̼�Ͻ𣨿�������̼���ֵ��ʵĻ���������������������̽��Ũ�����ijЩ���ʣ��������ͼ��ʾ��װ�ã��г�������ʡ�ԣ��ͷ�������ʵ�飮
ij��ѧ��ȤС��Ϊ�˲ⶨij��̼�Ͻ𣨿�������̼���ֵ��ʵĻ���������������������̽��Ũ�����ijЩ���ʣ��������ͼ��ʾ��װ�ã��г�������ʡ�ԣ��ͷ�������ʵ�飮��ش��������⣺
��1���ڼ��������£�A��̼��Ũ���ᷢ����Ӧ�Ļ�ѧ����ʽ��
��2���������װ�������Ե�һ�ַ����ǣ��رշ�Һ©���Ļ�������E��������һ�����ܣ�Ȼ��
��3�����ȳ���E������������ag��̼�Ͻ���Ʒ����װ��A�У��ټ�������Ũ���ᣬ��A�в����ݳ�����ʱ��ֹͣ���ȣ�����E�����أ����E����bg������̼�Ͻ���������������Ϊ
��4��װ��C������
��5����ͬѧ��Ϊ�����ݴ�ʵ���õ����ݣ�����Ͻ������������������ܻ�ƫ�ͣ�ԭ���ǿ����е�CO2��H2O����E������Ϊ�Ľ��ķ�����
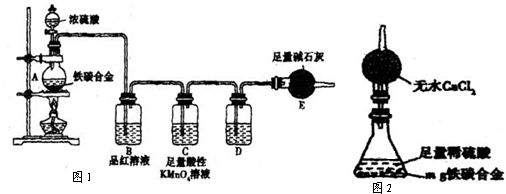
��������1������������Ũ�����жۻ���̼��Ũ�����ڼ��������²��ܷ�Ӧ��
��2������װ���е�ѹǿ�仯����Һ��仯�������飬������ܲ���ˮ�����ȷ���װ�õ�����ð���ݣ�ֹͣ��������һ��ˮ����
��3������mg��̼�Ͻ𣬼������Ũ���ᣬE����bg�������ɶ�����̼������Ϊbg�����������غ㶨�ɣ�������mg��̼�Ͻ��к�̼Ԫ�ص��������������������������
��4��װ��C�����ø��������Һ��ǿ���������ն�������
��5��Eװ�ú�������һ��ʢ��ʯ�ҵĸ���ܷ�ֹ�����еĶ�����̼��ˮ�����������ܣ�װ�������ɵĶ�����̼����ȫ������ʯ�����գ�
��2������װ���е�ѹǿ�仯����Һ��仯�������飬������ܲ���ˮ�����ȷ���װ�õ�����ð���ݣ�ֹͣ��������һ��ˮ����
��3������mg��̼�Ͻ𣬼������Ũ���ᣬE����bg�������ɶ�����̼������Ϊbg�����������غ㶨�ɣ�������mg��̼�Ͻ��к�̼Ԫ�ص��������������������������
��4��װ��C�����ø��������Һ��ǿ���������ն�������
��5��Eװ�ú�������һ��ʢ��ʯ�ҵĸ���ܷ�ֹ�����еĶ�����̼��ˮ�����������ܣ�װ�������ɵĶ�����̼����ȫ������ʯ�����գ�
����⣺��1��̼����Ũ������ȷ�Ӧ���ɶ�����̼�����������ˮ����Ӧ�Ļ�ѧ����ʽΪ��C+2H2SO4��Ũ��
CO2��+2SO2��+2H2O��
�ʴ�Ϊ��C+2H2SO4��Ũ��
CO2��+2SO2��+2H2O��
��2���رշ�Һ©���Ļ�������Eװ�ú�������һ�����ܣ�Ȼ���������ܲ���ˮ�����ȷ���װ�õ�����ð���ݣ�ֹͣ��������һ��ˮ��֤��װ�������Ժã�
�ʴ�Ϊ���������ܲ���ˮ�У�������ƿ��������ð���ݣ�ֹͣ������һ��ˮ��������
��3����ȡag��̼�Ͻ𣬼������Ũ���ᣬ���ȴ�A�в����ݳ�����ʱ��ֹͣ���ȣ�����Eװ�ò����أ�E����bg�������ɶ�����̼������Ϊbg�����������غ㶨�ɣ���mg��̼�Ͻ��к�̼Ԫ�ص�����Ϊ
=
g������������Ϊag-
g��������������Ϊ
��100%��
�ʴ�Ϊ��
��100%��
��4��װ��C�����ø��������Һ��ǿ���������ն������������ӷ�ӦΪ5SO2+2MnO4-+2H2O=5SO42-+2Mn2++4H+��
�ʴ�Ϊ�����ճ�ȥ��������5SO2+2MnO4-+2H2O=5SO42-+2Mn2++4H+��
��5��E����װ�Լ�Ϊ��ʯ�ң������տ�����CO2��H2Oʹb���Ľ��ķ���������һ��ʢ��ʯ�ҵĸ���ܷ�ֹ�����еĶ�����̼��ˮ���룻װ�������ɵĶ�����̼����ȫ������ʯ�����ջᵼ�²ⶨ���ƫ�ͣ�
�ʴ�Ϊ��Eװ�ú�������һ��ʢ��ʯ�ҵĸ���ܣ���Ӧ������CO2����δ����ȫ�ŵ�װ��E�У�����bƫ�ͣ�
| ||
�ʴ�Ϊ��C+2H2SO4��Ũ��
| ||
��2���رշ�Һ©���Ļ�������Eװ�ú�������һ�����ܣ�Ȼ���������ܲ���ˮ�����ȷ���װ�õ�����ð���ݣ�ֹͣ��������һ��ˮ��֤��װ�������Ժã�
�ʴ�Ϊ���������ܲ���ˮ�У�������ƿ��������ð���ݣ�ֹͣ������һ��ˮ��������
��3����ȡag��̼�Ͻ𣬼������Ũ���ᣬ���ȴ�A�в����ݳ�����ʱ��ֹͣ���ȣ�����Eװ�ò����أ�E����bg�������ɶ�����̼������Ϊbg�����������غ㶨�ɣ���mg��̼�Ͻ��к�̼Ԫ�ص�����Ϊ
| 12b |
| 44 |
| 3b |
| 11 |
| 3b |
| 11 |
| 11a-3b |
| 11a |
�ʴ�Ϊ��
| 11a-3b |
| 11a |
��4��װ��C�����ø��������Һ��ǿ���������ն������������ӷ�ӦΪ5SO2+2MnO4-+2H2O=5SO42-+2Mn2++4H+��
�ʴ�Ϊ�����ճ�ȥ��������5SO2+2MnO4-+2H2O=5SO42-+2Mn2++4H+��
��5��E����װ�Լ�Ϊ��ʯ�ң������տ�����CO2��H2Oʹb���Ľ��ķ���������һ��ʢ��ʯ�ҵĸ���ܷ�ֹ�����еĶ�����̼��ˮ���룻װ�������ɵĶ�����̼����ȫ������ʯ�����ջᵼ�²ⶨ���ƫ�ͣ�
�ʴ�Ϊ��Eװ�ú�������һ��ʢ��ʯ�ҵĸ���ܣ���Ӧ������CO2����δ����ȫ�ŵ�װ��E�У�����bƫ�ͣ�
���������⿼�����������ʵ�̽��ʵ�鷽����װ�õ��������������̷�Ӧ���Լ������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ

 ��5��������ҵ����ۣ�����������ͼװ�ã���������Լ�Ϊ��ˮ�Ȼ��ƣ���ƿ��Ϊmg��̿�Ͻ������ϡ���ᣮ������������ʵ�������ⶨijЩ���ݼ��ɣ�Ϊ�˿��ٲ�����������������������ʵ�������
��5��������ҵ����ۣ�����������ͼװ�ã���������Լ�Ϊ��ˮ�Ȼ��ƣ���ƿ��Ϊmg��̿�Ͻ������ϡ���ᣮ������������ʵ�������ⶨijЩ���ݼ��ɣ�Ϊ�˿��ٲ�����������������������ʵ�������