��Ŀ����
ʵ���ó�����0.1 mol/LijһԪ��(HA)��Һ��pH������l��0.1 mol/LijһԪ��(BOH)��Һ�C��H+��/C��OH����=10��12������������Һ�������Ϻ�������Һ������ӵ����ʵ���Ũ�ȵĹ�ϵ��ȷ����
- A.C��B+��>C(A��)>C(OH��)>C(H+) ������
- B.C(A��)>C(B+)>C(H+)>C(OH��)
- C.C(B+)+C (H+)��C(A��)+C(OH��) ����
- D.C(B+)>C(A��)>C(H+)>c(OH��)
AC
�ɳ�����0.1 mol/LijһԪ��(HA)��Һ��pH������l��֪HAΪ���ᣬ��0.1 mol/LijһԪ��(BOH)��Һ�C��H+��/C��OH����=10��12�ɵ�BOHΪһԪǿ������ߵ������Ϻ�ǡ����ȫ��Ӧ����BA��Һ������Һ����������ʵ���Ũ�ȵĹ�ϵΪ��C��B+��>C(A��)>C(OH��)>C(H+)���ָ��ݵ���غ��C(B+)+C (H+)��C(A��)+C(OH��)
�ɳ�����0.1 mol/LijһԪ��(HA)��Һ��pH������l��֪HAΪ���ᣬ��0.1 mol/LijһԪ��(BOH)��Һ�C��H+��/C��OH����=10��12�ɵ�BOHΪһԪǿ������ߵ������Ϻ�ǡ����ȫ��Ӧ����BA��Һ������Һ����������ʵ���Ũ�ȵĹ�ϵΪ��C��B+��>C(A��)>C(OH��)>C(H+)���ָ��ݵ���غ��C(B+)+C (H+)��C(A��)+C(OH��)

��ϰ��ϵ�д�
�����Ŀ
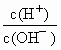 ��10��12������������Һ�������Ϻ�������Һ������ӵ����ʵ���Ũ���ɴ�С˳����
��10��12������������Һ�������Ϻ�������Һ������ӵ����ʵ���Ũ���ɴ�С˳����