��Ŀ����
��1.000gFeC2O4��2H20������Ʒ�������ط������н������ط������y�������ط������ߣ���Ʒ�������¶ȱ仯�����ߣ�����ͼ��ʾ��
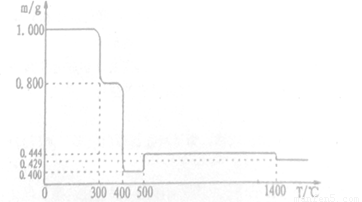
��֪���ٲ��������ȷֽ��ų�̼���������5000C֮ǰ�������ط����ǵ���Ʒ�ش��� Ar�������У�5000Cʱ����Ʒ���������ͨ�����������գ�
(1) 3000Cʱ����Ʒ��ˮ�Ĺ��̣���ȷ��3500Cʱ��Ʒ�Ƿ���ˮ��ȫ_____ (���"�� �����жϵ�������____(Ҫ��д��������̣���
(2 ) 4000Cʱ�����仯�Ļ�ѧ����ʽ��_______��
(3) ��6000C ʱ��Ʒ���в����Ĺ������������ȴ�����£�����ù����м���һ������ϡ����պ���ȫ�ܽ⣬��pH��ֽ���������Һ��PH��3����ԭ����____(�����ӷ���ʽ�ش𣩣������Һ�еμ�����NaOH��Һ���ɺ��ɫ�������y�ô�ʱ��Һ����Ԫ��������Ũ��Ϊ4.0x10-11 mol/L�����ʱ��Һ��pH�� _______(��֪��Ksp[Fe(OH)2]��8.0��10��16��Ksp[Fe(OH)3]��4.0��10��38)
(4) ��15000Cʱ��Ʒ���в����Ĺ������������ȴ����ϡ�����ܽ��һ�ػ�ɫ��Һ�� ȡ��������Һ�μ�KSCN,��Һ�Ժ�ɫ����ȡ��������Һ�μ�K3[Fe(CN)6)(���軯�أ���Һ������������ɫ��������д��ͼ��14000Cʱ������Ӧ�Ļ�ѧ����ʽ_________ ,����������ɫ���������ӷ�Ӧ����ʽ______��
��1���ǣ�2�֣���2��FeC2O4 FeO��CO����CO2����2�֣�
FeO��CO����CO2����2�֣�
��3��Fe3����3OH��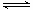 Fe(OH)3��3H����2�֣� �� 5��2�֣� ��4��6Fe2O3
Fe(OH)3��3H����2�֣� �� 5��2�֣� ��4��6Fe2O3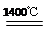 4Fe3O4��O2����2�֣���
4Fe3O4��O2����2�֣���
3Fe2����2[Fe(CN)6]3����Fe3[Fe(CN)6]2����K����Fe2����[Fe(CN)6]3����KFe[Fe(CN)6]����2�֣�
��������
�����������1����Ʒ�ֽ�ķ���ʽ��
FeC2O4��2H2O FeC2O4��(2��n)H2O��nH2O
FeC2O4��(2��n)H2O��nH2O
180g 18n
1.000g ��1.000g��0.800g��
���n��2��������Ʒ�Ѿ���ȫ�ֽ�
��2��400��ʱ�����ּ�����0.800g��0.400g��0.400g
������ԭ���غ��֪��400�����ĺ���Ӧ����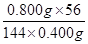 ��
��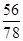 ����˸û������������������仯ѧʽ��FeO�����Է�Ӧ�Ļ�ѧ����ʽ��FeC2O4
����˸û������������������仯ѧʽ��FeO�����Է�Ӧ�Ļ�ѧ����ʽ��FeC2O4 FeO��CO����CO2����
FeO��CO����CO2����
��3��600����Ʒ����Ԫ�صĺ�����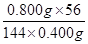 ��0.70����˸ù��������������������������������Ȼ�����������ˮ����Һ�����ԣ������ӷ���ʽ��Fe3����3OH��
��0.70����˸ù��������������������������������Ȼ�����������ˮ����Һ�����ԣ������ӷ���ʽ��Fe3����3OH�� Fe(OH)3��3H�������������������ܶȻ�������֪������Һ�������ӵ�����Ũ��Ϊ4.0x10-11 mol/Lʱ����Һ��c(OH��)��
Fe(OH)3��3H�������������������ܶȻ�������֪������Һ�������ӵ�����Ũ��Ϊ4.0x10-11 mol/Lʱ����Һ��c(OH��)�� ��10��9mol/L��������Һ��c(H��)��10��5mol/L�����pH��5��
��10��9mol/L��������Һ��c(H��)��10��5mol/L�����pH��5��
��4����ȡ��������Һ�μ�K3[Fe(CN)6)(���軯�أ���Һ������������ɫ��������˵����Һ�л��������������ɡ����1400��ʱ�������ֽ���������������������Ӧ�Ļ�ѧ����ʽ��6Fe2O3 4Fe3O4��O2��������������ɫ���������ӷ�Ӧ����ʽ3Fe2����2[Fe(CN)6]3����Fe3[Fe(CN)6]2����K����Fe2����[Fe(CN)6]3����KFe[Fe(CN)6]����
4Fe3O4��O2��������������ɫ���������ӷ�Ӧ����ʽ3Fe2����2[Fe(CN)6]3����Fe3[Fe(CN)6]2����K����Fe2����[Fe(CN)6]3����KFe[Fe(CN)6]����
���㣺������������ֽ���йؼ��㡢���Ӽ����Լ�����ʽ����д��



 ���к͵ζ����ⶨij�ռ��Ũ�ȣ���ش���Ҫ���������е��й����⣺
���к͵ζ����ⶨij�ռ��Ũ�ȣ���ش���Ҫ���������е��й����⣺