��Ŀ����
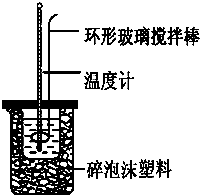 ʵ������50mL 0.50mol?L-1���ᡢ50mL 0.55mol?L-1 NaOH��������ͼװ�ý����к��ȵIJⶨ����ش��������⣺
ʵ������50mL 0.50mol?L-1���ᡢ50mL 0.55mol?L-1 NaOH��������ͼװ�ý����к��ȵIJⶨ����ش��������⣺��1��������ͭ˿��������滷�β����������������
��2������0.50mol?L-1������NaOH�������ʵ�飬��ʵ���в�õġ��к��ȡ���ֵ��
��3��ʵ��õ����е����ݣ�
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
| ���� | NaOH��Һ | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |
[�����NaOH��Һ���ܶȰ�1g?cm-3���㣬��Ӧ������Һ�ı����ݣ�c����4.18J?��g?�棩-1����]��
��������1���������ȿ죬������ʧ�ࣻ
��2���������ƹ�������ˮ���ȣ���Һ�¶����ߣ�
��3���ȸ��ݹ�ʽQ=cm��T���������0.025mol��ˮ�ų�������Ȼ������к��ȵĸ�������к��ȣ�
��2���������ƹ�������ˮ���ȣ���Һ�¶����ߣ�
��3���ȸ��ݹ�ʽQ=cm��T���������0.025mol��ˮ�ų�������Ȼ������к��ȵĸ�������к��ȣ�
����⣺��1�����ܽ����β����������Ϊͭ˿���������Ϊͭ˿��������ȵ������壬
�ʴ�Ϊ��Cu���ȿ죬������ʧ��
��2���������ƹ�������ˮ���ȣ���Һ�¶����ߣ�����ʵ���в�õġ��к��ȡ���ֵ��ƫ��
�ʴ�Ϊ��ƫ��
��3��50mL 0.50mol?L-1���ᡢ50mL 0.55mol?L-1 NaOH��������Ϊm=100mL��1g/mL=100g��c=4.18J/��g?�棩����T=t2-t1=3.4�棬���빫ʽQ=cm��T������0.025mol��ˮ�ų�����Q=4.18J/��g?�棩��100g��3.4��=1421.2J=1.4212KJ��������0.025mol��ˮ�ų�����1.4212KJ����������1mol��ˮ�ų�����Ϊ
=56.8kJ������ʵ���õ��к��ȡ�H=-56.8kJ/mol��
�ʴ�Ϊ��-56.8kJ/mol��
�ʴ�Ϊ��Cu���ȿ죬������ʧ��
��2���������ƹ�������ˮ���ȣ���Һ�¶����ߣ�����ʵ���в�õġ��к��ȡ���ֵ��ƫ��
�ʴ�Ϊ��ƫ��
��3��50mL 0.50mol?L-1���ᡢ50mL 0.55mol?L-1 NaOH��������Ϊm=100mL��1g/mL=100g��c=4.18J/��g?�棩����T=t2-t1=3.4�棬���빫ʽQ=cm��T������0.025mol��ˮ�ų�����Q=4.18J/��g?�棩��100g��3.4��=1421.2J=1.4212KJ��������0.025mol��ˮ�ų�����1.4212KJ����������1mol��ˮ�ų�����Ϊ
| 1.4212KJ��1mol |
| 0.025mol |
�ʴ�Ϊ��-56.8kJ/mol��
���������⿼���к��ȵIJⶨ����Ŀ�ѶȲ���ע�������к��ȵĸ����Լ��������㹫ʽ��Ӧ����������λ�Ļ��㣮

��ϰ��ϵ�д�
�����Ŀ
 ʵ������50mL 0.50mol?L-1���ᡢ50mL 0.55mol?L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�
ʵ������50mL 0.50mol?L-1���ᡢ50mL 0.55mol?L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�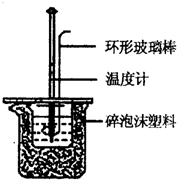 ʵ������50mL 0.50mol/L���ᡢ50mL 0.55mol/L NaOH��Һ����ͼ��ʾװ�ý��вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�
ʵ������50mL 0.50mol/L���ᡢ50mL 0.55mol/L NaOH��Һ����ͼ��ʾװ�ý��вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�