��Ŀ����
����ת����ϵ�У�X��Y����������;�㷺�����ֽ������ʣ�A��B�������A�ʺ���ɫ��C��D��E����ѧ���������ֻ��������ת����ϵ�ش����⣺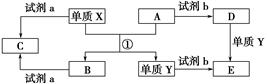
��1����д����Ӧ�ٵĻ�ѧ����ʽ___________________________________________��
��2������D��Һ��Y���ӵķ�����_____________________________________��
��3�����Լ�a��NaOH��Һ��д������X��NaOH��Һ��Ӧ�����ӷ���ʽ______________________________��
��4�����Լ�b��H2SO4����ҵ����E��H2SO4��NaNO2Ϊԭ����ȡ��Ч��ˮ��Y��OH��SO4����֪��ԭ����ΪNO����÷�Ӧ�Ļ�ѧ����ʽ��_____________________________________________________________________��
��5����ҵ�ϵ�����ڵ�B��ȡXʱ�������������������ڱ�״���µ����Ϊ33.6 m3�����������������Ϊ________kg��
��1��Fe2O3��2Al 2Fe��Al2O3
2Fe��Al2O3
��2��ȡ����D��Һ���Թ��У��μӼ���KSCN��Һ������Һ���ɫ����֤����Fe3��
��3��2Al��2OH����2H2O=2AlO2-��3H2��
��4��2FeSO4��H2SO4��2NaNO2=2Fe��OH��SO4��Na2SO4��2NO��
��5��54
����

 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д����ȷ�Ӧ������ұ�����۵�Ľ������ɼ���Ϊ������ijЩ�����������ڸ��������·����ķ�Ӧ��ijѧϰС������ȷ�Ӧ����Al��Fe2O3��ӦΪ����ʵ������о���
�������ݵõ�Al��Al2O3��Fe��Fe2O3���۵㡢�е��������±���ʾ��
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1565[ |
| �е�/�� | 2467 | 2980 | 2750 | �� |
�Իش��������⣺
��1�������ȷ�Ӧ�н��������ֳ� �ԣ����������ԭ���������ж����н�������һ�����������ȷ�Ӧ��ȡ ��������ţ�
��Fe����Cr����������V����������Ca����Mn
��2��ijͬѧ�Ʋ⣬���ȷ�Ӧ���õ�����������Ӧ�������������ֽ����������һ����ʵ�鷽��֤�����������к��н���������ʵ�������õ��Լ�Ϊ ���ɹ۲쵽��ʵ�������� ��
��3����һͬѧ�Ʋ����ȷ�Ӧ�õ����������л�����Fe2O3������������·�������֤��
ȡһ���������Ͷ�뵽����ϡ�����У���Ӧ��Ļ��Һ�еμ����ʼ���Һ���۲���Һ��ɫδ��Ѫ��ɫ������֤���������в�����Fe2O3����
�����ʼ��� ���ѧʽ����
�ڸ�ͬѧ��ʵ�鷽���Ƿ������ ���������������������
���ɣ�
�Ի�ͭ����Ҫ�ɷ�ΪCuFeS2������������SiO2�ȣ�Ϊԭ�Ͻ�����ͭ��ͬʱ�õ�����Ʒ�̷���FeSO4��7H2O��������Ҫ�������£�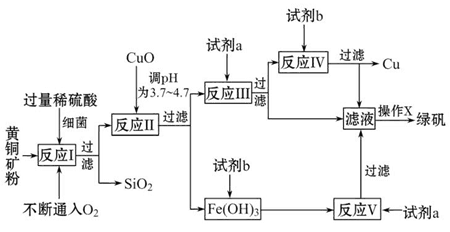
��֪���� 4CuFeS2+2H2SO4+17O2=4CuSO4+2Fe2��SO4��3+2H2O
�ڲ���������������������ʽ��ʼ��������ȫ����ʱ��Һ��pH���
| ������ | Cu��OH��2 | Fe��OH��3 | Fe��OH��2 |
| ��ʼ����pH | 4��7 | 2��7 | 7��6 |
| ��ȫ����pH | 6��7 | 3��7 | 9��6 |
��1���Լ�a��__________���Լ�b��__________��
��2������XӦΪ����Ũ����__________��__________��
��3����Ӧ���м�CuO��pHΪ__________��Ŀ����ʹFe3+�γ�Fe��OH��3��������ֹ����Cu��OH��2������
��4����Ӧ�������ӷ���ʽΪ��__________________________________________��
 4Al+3O2���������ʯ������:_______________________________��
4Al+3O2���������ʯ������:_______________________________��
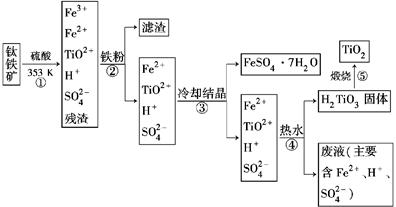
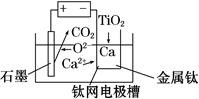
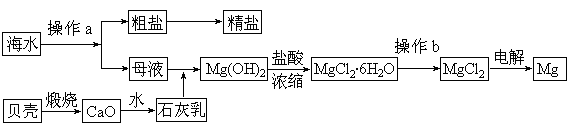
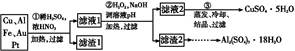
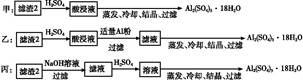
 CuY2-+2H+��д������CuSO4��5H2O���������ı���ʽw=�� ;
CuY2-+2H+��д������CuSO4��5H2O���������ı���ʽw=�� ;  ��ϡ��Һ�У���ʹ���ַ�Ӧ��������Ļ�ԭ����ֻ��NO���Իش��������⣺
��ϡ��Һ�У���ʹ���ַ�Ӧ��������Ļ�ԭ����ֻ��NO���Իش��������⣺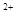 ��Cu
��Cu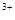 ��Fe
��Fe