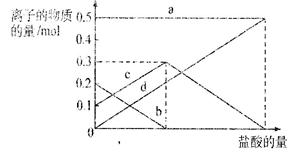
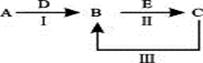
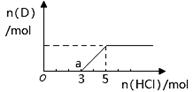
��Ŀ����
��������ҵ���������ɡ���������ȡ������ұ�������ļӹ��Ȼ��ڹ��ɡ���ش���������:
��1����ҵ�ϲ��õ��������-����ʯ(Na3AlF6)������ķ���ұ���õ�������:
2Al2O3 4Al+3O2���������ʯ������:_______________________________��
4Al+3O2���������ʯ������:_______________________________��
��2��������������������������������Fe��Si������,���õ�ⷽ����һ���ᴿ,�õ����������ĵ缫��ӦʽΪ������ ��,���п����������ϵ���________________��
A.���ġ� ��B.ʯī�� ��C.Ǧ�塡�� D.����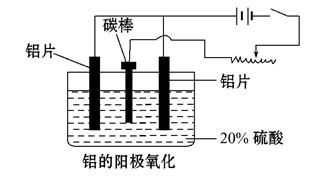
��3������������ʹ���������������ܵ�����Ĥ����ϡ����Ϊ���Һ,�����������ĵ缫��ӦʽΪ__________________________________��
��4��������������������,��Ҫ���ϵص�����ѹ,������____________ ��
��5������˵����ȷ��������������
A. ����������Ӧ��ԭ���ԭ�����н������ϱ��洦���ļ���
B. ����������������ǿ������ľ�Ե����
C. ����������������߽���������Ͻ����ʴ��,����ĥ���½�
D. ������������Ĥ���ж����,���к�ǿ����������,������Ⱦ�϶��ʸ�����ɫ
��1������Al2O3�۵� ��2�֣�
��2��Al- 3e-= Al3+��2�֣� D��2�֣�
��3��2 Al- 6e-+ 3H2O = Al2O3 + 6H+��2�֣�
��4�����������治�������������������Ϊ�˱����ȶ��ĵ�������Ҫ���������ѹ����3�֣�
��5��B��D��4�֣�
���������������1���������۵�ϸߣ����ʱ�ܺĴ������ʯ���Խ���Al2O3�ۻ����¶ȣ������ܺģ���2����Ͽα���ѧ����ͭ�ƾ�ͭ����ͭ����������ͭ��������֪����������������������Ӧ���缫����ʽΪ;Al- 3e-= Al3+,������������ѡ��D�𰸣���3��������������������Ӧ�γ����������缫��ӦΪ��2Al- 6e-+ 3H2O = Al2O3 + 6H+����4�����������治�������������������Ϊ�˱����ȶ��ĵ�������Ҫ���������ѹ����5��B��D
���㣺����绯ѧԭ�����缫����ʽ����д��

 ��������һ���þ�ϵ�д�
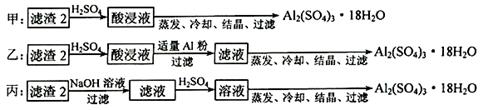
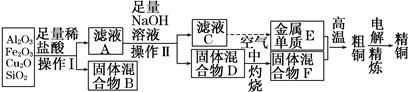
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�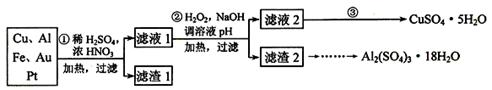
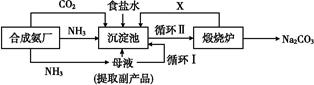

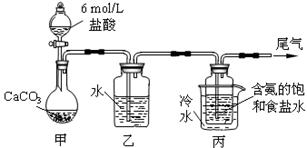
 ij��ѧС��ģ�⡰�����Ƽ������NaCl��NH3��CO2��ˮ��Ϊԭ���Լ���ͼ��ʾװ
ij��ѧС��ģ�⡰�����Ƽ������NaCl��NH3��CO2��ˮ��Ϊԭ���Լ���ͼ��ʾװ