��Ŀ����
ij��ȤС��Ϊ�о�������������ȡ��ˮ�⣬��������ʵ�飮ʵ��һ ���Ҵ���������ȡ��������
�ס���ͬѧ�ֱ�ʹ��ͼ1��ͼ2װ����ȡ����������

��1����ͬѧ���Ƶ��������������������뱥��̼������Һ�����������
��2����ȡ���������Ļ�ѧ����ʽΪ
��3��ͼ1����ĩ�˲��ܲ��뱥��̼������Һ����ͼ2װ���е�����C��ĩ�˿��Բ��뱥��̼������Һ��ԭ����
ʵ��� �ⶨ���������Ĵ���
ȡ��ͬѧ��������������Ʒ1.0g������20.00mL 0.500mol?L-1��NaOH��Һ�У��������Сʱ���������η�̪����0.075mol?L-1���������Һ�ζ����ظ�����ʵ��������������ƽ��ֵΪ20.00mL��
��4��������������Ʒ�Ĵ���Ϊ
��5�����к͵ζ�ȫ����û�������ʵ������
��Դ������
ʵ���� �ⶨ��������ˮ�ⷴӦ��ƽ�ⳣ��
��25�棬ȡ��ͬѧ��������������Ʒ10.0ml����1000mL 0.005mol?L-1 NaOH��Һ�У�������㹻��ʱ�䣬ʹ��Ӧ�ﵽƽ��״̬�����ã�
��6�����ˮ����
��7����Ҫ�ó�ͼ3���ߣ�Ӧ���ڲ�ͬ
��������1���������������ͱ���̼������Һ�������ܣ����÷�Һ�ķ������룻
��2������������Ӧ�ı���Ϊ�����ǻ��������⣬�÷�Ӧ��������������ˮ����Ϊ���淴Ӧ��
��3����������C�Ϸ���һ�ϴ��ݻ��Ŀռ䣬Һ�岻���ܷ���������
��4���ȸ��ݹ�ϵʽ��NaOH��HCl����������������ƣ��Ӷ����������������Ӧ���������Ƶ����ʵ�����Ȼ����ݹ�ϵʽ��CH3COOCH2CH3��NaOH�������������Ȼ���������������Ʒ�Ĵ��ȣ�
��5����������δ��ȫˮ�⣻
��6������ƽ�ⳣ������������Ũ����֮���뷴Ӧ��Ũ����֮���ıȣ�
��7������ͼ3���߱�ʾ��ͬ�¶�������ƽ��Ũ�ȣ�ÿ���������ٲⶨ2�Σ�
��2������������Ӧ�ı���Ϊ�����ǻ��������⣬�÷�Ӧ��������������ˮ����Ϊ���淴Ӧ��
��3����������C�Ϸ���һ�ϴ��ݻ��Ŀռ䣬Һ�岻���ܷ���������
��4���ȸ��ݹ�ϵʽ��NaOH��HCl����������������ƣ��Ӷ����������������Ӧ���������Ƶ����ʵ�����Ȼ����ݹ�ϵʽ��CH3COOCH2CH3��NaOH�������������Ȼ���������������Ʒ�Ĵ��ȣ�
��5����������δ��ȫˮ�⣻
��6������ƽ�ⳣ������������Ũ����֮���뷴Ӧ��Ũ����֮���ıȣ�
��7������ͼ3���߱�ʾ��ͬ�¶�������ƽ��Ũ�ȣ�ÿ���������ٲⶨ2�Σ�
����⣺��1�����������ͱ���̼������Һ�������ܣ����÷�Һ�ķ������룬�õ�������Ϊ��Һ©����
�ʴ�Ϊ����Һ©����
��2��������Ӧ�ı���Ϊ�����ǻ��������⣬�������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ����ѧ����ʽΪ��CH3CH2OH+CH3COOH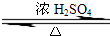 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
�ʴ�Ϊ��CH3CH2OH+CH3COOH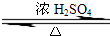 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��3������C�Ϸ���һ�ϴ��ݻ��Ŀռ䣬Һ�岻�ᵹ����A��
�ʴ�Ϊ������C�Ϸ���һ�ϴ��ݻ��Ŀռ䣬Һ�岻�ᵹ����A��
��4��20.00mL 0.500mol?L-1��NaOH��Һ���������Ƶ����ʵ���Ϊ20.00mL 0.500mol?L-1��0.02L=0.01mol���ɹ�ϵʽ��NaOH��HCl�����������������Ϊ0.075mol?L-1��0.02L=0.0015mol����������������Ӧ���������Ƶ����ʵ���Ϊ0.01mol-0.0015mol=0.0085mol���ɹ�ϵʽ��CH3COOCH2CH3��NaOH�����������Ϊ0.0085mol��������Ϊ0.0085mol��88g/mol=0.748g������������Ʒ�Ĵ���Ϊ
��100%=74.8%��
�ʴ�Ϊ��=74.8%��
��5�����к͵ζ�ȫ����û�������ʵ��������Դ��������������δ��ȫˮ�⣻
�ʴ�Ϊ������������δ��ȫˮ�⣻
��6������ƽ�ⳣ������������Ũ����֮���뷴Ӧ��Ũ����֮���ıȣ���֪�������������������Ƶ����ʵ���Ũ�ȣ������������ƺ��Ҵ���Ũ�ȣ��Ӷ��������������ˮ�ⷴӦ��ƽ�ⳣ����
�ʴ�Ϊ�������������������Ƶ����ʵ���Ũ�ȣ�
��7��ͼ3���߱�ʾ��ͬ�¶�������ƽ��Ũ�ȣ�ÿ���������ٲⶨ2�Σ����ٹ�����10�Σ�
�ʴ�Ϊ���¶ȣ�10��
�ʴ�Ϊ����Һ©����
��2��������Ӧ�ı���Ϊ�����ǻ��������⣬�������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ����ѧ����ʽΪ��CH3CH2OH+CH3COOH
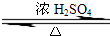 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O���ʴ�Ϊ��CH3CH2OH+CH3COOH
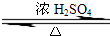 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O����3������C�Ϸ���һ�ϴ��ݻ��Ŀռ䣬Һ�岻�ᵹ����A��
�ʴ�Ϊ������C�Ϸ���һ�ϴ��ݻ��Ŀռ䣬Һ�岻�ᵹ����A��
��4��20.00mL 0.500mol?L-1��NaOH��Һ���������Ƶ����ʵ���Ϊ20.00mL 0.500mol?L-1��0.02L=0.01mol���ɹ�ϵʽ��NaOH��HCl�����������������Ϊ0.075mol?L-1��0.02L=0.0015mol����������������Ӧ���������Ƶ����ʵ���Ϊ0.01mol-0.0015mol=0.0085mol���ɹ�ϵʽ��CH3COOCH2CH3��NaOH�����������Ϊ0.0085mol��������Ϊ0.0085mol��88g/mol=0.748g������������Ʒ�Ĵ���Ϊ
| 0.748g |
| 1g |
�ʴ�Ϊ��=74.8%��
��5�����к͵ζ�ȫ����û�������ʵ��������Դ��������������δ��ȫˮ�⣻
�ʴ�Ϊ������������δ��ȫˮ�⣻
��6������ƽ�ⳣ������������Ũ����֮���뷴Ӧ��Ũ����֮���ıȣ���֪�������������������Ƶ����ʵ���Ũ�ȣ������������ƺ��Ҵ���Ũ�ȣ��Ӷ��������������ˮ�ⷴӦ��ƽ�ⳣ����
�ʴ�Ϊ�������������������Ƶ����ʵ���Ũ�ȣ�
��7��ͼ3���߱�ʾ��ͬ�¶�������ƽ��Ũ�ȣ�ÿ���������ٲⶨ2�Σ����ٹ�����10�Σ�
�ʴ�Ϊ���¶ȣ�10��
���������⿼�������������Ʊ����ⶨ���������Ĵ��ȣ��ⶨ��������ˮ�ⷴӦ��ƽ�ⳣ�������ʱ���շ�Ӧԭ�����ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
��12�֣�ij��ȤС��Ϊ�о�������������ȡ����������ʵ�顣
ʵ��һ ���Ҵ���������ȡ��������
�ס���ͬѧ�ֱ�ʹ��ͼ1��ͼ2װ����ȡ����������
ͼ1 ͼ2
��1����ȡ���������Ļ�ѧ����ʽΪ
��2��������̼������Һ��������������Һ�Ƿ���У�������ԭ��
��3��ͼ1����ĩ�˲��ܲ��뱥��̼������Һ����ͼ2װ���е�����C��ĩ�˿��Բ��뱥��̼������Һ��ԭ����
ʵ��� �ⶨ���������Ĵ���
ȡ��ͬѧ��������������Ʒ1.0 g������20.00 mL 0.500 mol��L-1��NaOH��Һ�У��������Сʱ���������η�̪����0.075 mol��L-1���������Һ�ζ����ظ�����ʵ��������������ƽ��ֵΪ20.00 mL��
��4��������������Ʒ�Ĵ���Ϊ
��5���ﵽ�ζ��յ�ʱ����Һ��ɫ�� ��Ϊ
��6�����в���������������������ƫ�͵�ԭ����
| A����ʽ�ζ���δ��ϴ | B����ʽ�ζ��ܵζ�ǰ�����ݣ��ζ���������ʧ |
| C�����������Һʱ�����ݸ��� | D����������δ��ȫˮ�� |
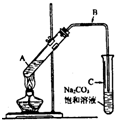 ��2009?�ɶ�һģ��ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о���
��2009?�ɶ�һģ��ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о��� CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O
