��Ŀ����
ʵ������Ҫ0.80mol?L-1 NaOH��Һ475mL0.40mol?L-1����500mL��������������Һ����������ش��������⣺
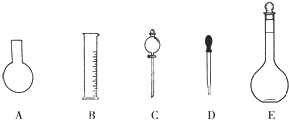
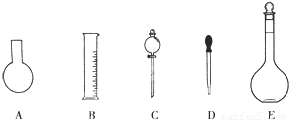
��1����ͼ��ʾ��������������Һ�϶�����Ҫ����
��2�����в����У�����ƿ�����߱��Ĺ�����
A������һ�����ȷŨ�ȵı���Һ
B����ȡһ�������Һ��
C����������ƿ������µ����������Һ��
D��������Һ
E�����������ܽ��������
��3�����ݼ�����������ƽ��ȡNaOH������Ϊ
��4�����ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84g?cm-3��Ũ��������Ϊ

��1����ͼ��ʾ��������������Һ�϶�����Ҫ����
AC
AC
������ţ�������������Һ�����õ��IJ����������ձ���������
�ձ���������
�����������ƣ�����2�����в����У�����ƿ�����߱��Ĺ�����
CDE
CDE
������ţ���A������һ�����ȷŨ�ȵı���Һ
B����ȡһ�������Һ��
C����������ƿ������µ����������Һ��
D��������Һ
E�����������ܽ��������
��3�����ݼ�����������ƽ��ȡNaOH������Ϊ
16.0
16.0
g������������һλС��������4�����ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84g?cm-3��Ũ��������Ϊ
10.9
10.9
mL������������һλС���������ʵ������10mL��15mL��20mL��50mL����Ͳ��Ӧѡ��15
15
mL����Ͳ��ã���������1������������Һ��ʵ���������ѡ������������
��2����������ƿ�Ľṹ�ص���ʹ��ԭ����
��3��ʵ��������475mL 0.5mol?L-1��NaOH��Һʱ��û��475mL����ƿ��ѡ�������Դ�����ӽ�������ƿ����Ӧѡ��500mL����ƿ������n=cv�����������Ƶ����ʵ������ٸ���m=nM���������������Ƶ�������
��4������c=
����Ũ�����Ũ�ȣ��ٸ���ϡ�Ͷ��ɼ�������Ũ��������������Ũ��������ѡ����ʵ���Ͳ��
��2����������ƿ�Ľṹ�ص���ʹ��ԭ����
��3��ʵ��������475mL 0.5mol?L-1��NaOH��Һʱ��û��475mL����ƿ��ѡ�������Դ�����ӽ�������ƿ����Ӧѡ��500mL����ƿ������n=cv�����������Ƶ����ʵ������ٸ���m=nM���������������Ƶ�������
��4������c=
| 1000�Ѧ� |
| M |
����⣺��1�����������м��㡢����������ȡ�����ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�������������ƽ�����������ƣ�����Ͳ��ȡŨ���ᣬ�õ���ͷ�ιܣ�����ҩ��ȡҩƷ�����ձ����ܽ⣨������Ͳ��ˮ�����ò��������裬�����ܽ⣬�ָ������º�ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ���ϴ��Һת�Ƶ�����ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
���������У�������ƽ����Ͳ��500mL����ƿ���ձ�������������ͷ�ιܡ�ҩ�ȣ�
�϶�����Ҫ���ǣ�Բ����ƿ����Һ©���������õ��IJ��������ǣ��ձ�����������
�ʴ�Ϊ��AC���ձ�����������
��2������ƿ�����������桢������Һ��ֻ��1���̶��߲��ܲ�������ƿ������µ����������Һ�壬��ѡ��CDE��
��3��û��475mL����ƿ��ѡ�������Դ�����ӽ�������ƿ����Ӧѡ��500mL����ƿ�������������Ƶ�����Ϊ0.5L��0.80mol?L-1��40g/mol=16.0g���ʴ�Ϊ��16.0��
��4����������Ϊ98%���ܶ�Ϊ1.84g?cm-3��Ũ��������ʵ���Ũ��Ϊ
mol/L=18.4mol/L��Ũ����ϡ��ǰ�����ʵ����ʵ������䣬����ҪŨ����������V����18.4mol/L��V=500mL��0.4mol/L�����V=10.9mL����ѡ��15mL����Ͳ��
�ʴ�Ϊ��10.9��15��
���������У�������ƽ����Ͳ��500mL����ƿ���ձ�������������ͷ�ιܡ�ҩ�ȣ�
�϶�����Ҫ���ǣ�Բ����ƿ����Һ©���������õ��IJ��������ǣ��ձ�����������
�ʴ�Ϊ��AC���ձ�����������
��2������ƿ�����������桢������Һ��ֻ��1���̶��߲��ܲ�������ƿ������µ����������Һ�壬��ѡ��CDE��
��3��û��475mL����ƿ��ѡ�������Դ�����ӽ�������ƿ����Ӧѡ��500mL����ƿ�������������Ƶ�����Ϊ0.5L��0.80mol?L-1��40g/mol=16.0g���ʴ�Ϊ��16.0��
��4����������Ϊ98%���ܶ�Ϊ1.84g?cm-3��Ũ��������ʵ���Ũ��Ϊ
| 1000��1.84��98% |
| 98 |
�ʴ�Ϊ��10.9��15��
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ��Ƚϻ�����ע���c=
��������ԭ���������������ƹ��̣�
| n |
| V |

��ϰ��ϵ�д�
�����Ŀ