��Ŀ����
��������AlN���������¡�������������Ժõ��������ʣ����㷺Ӧ���ڵ��ӹ�ҵ���մɹ�ҵ��������һ�������£���������ͨ�����·�Ӧ�ϳɣ�Al2O3+N2+3C=2AlN+3CO
��1��������˫�ŷ�������������ԭ��Ӧ�����������������ԭ�������ӵ�ʧ�������Լ����������ԭ���
Al2O3+N2+3C=2AlN+3CO______
��2����������һ�������»�����NaOH��Һ������Ӧ����NaAlO2��ƫ�����ƣ���NH3������a g����������������NaOH��Һ��ַ�Ӧ�����ɵİ���ȫ������b gˮ�У���ð�ˮ���ܶ�Ϊd����ð�ˮ�����ʵ���Ũ��Ϊ______mol/L����a��b��d��ʽ��ʾ���ɣ�
�⣺��1��Al2O3+N2+3C=2AlN+3CO��NԪ�صĻ��ϼ���0����Ϊ-3�ۣ�CԪ�صĻ��ϼ���0����Ϊ+2�ۣ�����Ϊ��������CΪ��ԭ����N�õ�2��3e-��Cʧȥ3��2e-��ת��6e-������������ԭ����ԭ����ΪAlN����ԭ��������������������ΪCO������ ��
��
�ʴ�Ϊ�� ��
��
��2����Nԭ���غ��֪��a g��������������ʵ���Ϊ =
= mol����n��NH3��=
mol����n��NH3��= mol����Һ������Ϊ
mol����Һ������Ϊ ��17g+bg��
��17g+bg��
��V=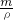 ��֪����Һ�����Ϊ
��֪����Һ�����Ϊ L��
L��
��c= =
= mol/L���ʴ�Ϊ��
mol/L���ʴ�Ϊ�� ��
��
��������1��Al2O3+N2+3C=2AlN+3CO��NԪ�صĻ��ϼ���0����Ϊ-3�ۣ�CԪ�صĻ��ϼ���0����Ϊ+2�ۣ��Դ������
��2����Nԭ���غ��֪��a g��������������ʵ���Ϊ =
= mol����n��NH3��=
mol����n��NH3��= mol����Һ������Ϊ
mol����Һ������Ϊ ��17g+bg����V=
��17g+bg����V=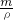 ��c=
��c= ���㣮
���㣮
���������⿼��������ԭ��Ӧ������㣬��ȷ��Ӧ��Ԫ�صĻ��ϼ۱仯��������ԭ���غ���ǽ����Ĺؼ���ע���������ͻ�����ʽ��Ӧ�ã���Ŀ�ѶȲ���
 ��
���ʴ�Ϊ��
 ��
����2����Nԭ���غ��֪��a g��������������ʵ���Ϊ
 =
= mol����n��NH3��=
mol����n��NH3��= mol����Һ������Ϊ
mol����Һ������Ϊ ��17g+bg��
��17g+bg����V=
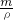 ��֪����Һ�����Ϊ
��֪����Һ�����Ϊ L��
L����c=
 =
= mol/L���ʴ�Ϊ��
mol/L���ʴ�Ϊ�� ��
����������1��Al2O3+N2+3C=2AlN+3CO��NԪ�صĻ��ϼ���0����Ϊ-3�ۣ�CԪ�صĻ��ϼ���0����Ϊ+2�ۣ��Դ������
��2����Nԭ���غ��֪��a g��������������ʵ���Ϊ
 =
= mol����n��NH3��=
mol����n��NH3��= mol����Һ������Ϊ
mol����Һ������Ϊ ��17g+bg����V=
��17g+bg����V=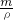 ��c=
��c= ���㣮
���㣮���������⿼��������ԭ��Ӧ������㣬��ȷ��Ӧ��Ԫ�صĻ��ϼ۱仯��������ԭ���غ���ǽ����Ĺؼ���ע���������ͻ�����ʽ��Ӧ�ã���Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
