��Ŀ����
��һ�ݻ�������ܱ������г���һ����A��B���������·�Ӧ��
x A��g�� +2B��s�� y C��g��; ��H <0��һ�������£�������A��C�����ʵ���Ũ����ʱ��仯����������ͼ����ش��������⣺
y C��g��; ��H <0��һ�������£�������A��C�����ʵ���Ũ����ʱ��仯����������ͼ����ش��������⣺

��1����A��Ũ�ȱ仯��ʾ�÷�Ӧ0��10min�ڵ�ƽ����Ӧ����v��A��= ��
��2������ͼʾ��ȷ��x��y= ��
��3��0��l0min������ѹǿ____ �����������䡱��С����
��4���Ʋ��l0min�������߱仯�ķ�Ӧ���������� ����16min�������߱仯�ķ�Ӧ����������____ ��
�ټ�ѹ��������A��Ũ�ȣ�������C�����������£��ݽ��£��Ӵ���
��5����ƽ��I��ƽ�ⳣ��ΪK1��ƽ���ƽ�ⳣ��ΪK2����K1 K2���>����=����<����
x A��g�� +2B��s��
 y C��g��; ��H <0��һ�������£�������A��C�����ʵ���Ũ����ʱ��仯����������ͼ����ش��������⣺
y C��g��; ��H <0��һ�������£�������A��C�����ʵ���Ũ����ʱ��仯����������ͼ����ش��������⣺
��1����A��Ũ�ȱ仯��ʾ�÷�Ӧ0��10min�ڵ�ƽ����Ӧ����v��A��= ��
��2������ͼʾ��ȷ��x��y= ��
��3��0��l0min������ѹǿ____ �����������䡱��С����
��4���Ʋ��l0min�������߱仯�ķ�Ӧ���������� ����16min�������߱仯�ķ�Ӧ����������____ ��
�ټ�ѹ��������A��Ũ�ȣ�������C�����������£��ݽ��£��Ӵ���
��5����ƽ��I��ƽ�ⳣ��ΪK1��ƽ���ƽ�ⳣ��ΪK2����K1 K2���>����=����<����
��10�֣���1��0.02mol/(L��min)(2��) ��2��1��2��2�֣� ��3�����1�֣�
��4���ܢޣ�2�֣� �ܣ�1�֣� ��5��> (2��)
��4���ܢޣ�2�֣� �ܣ�1�֣� ��5��> (2��)
�����������1��v��A��=(0.45mol/L-0.25mol/L)��10min=0.02mol/(L��min)��
��2������ͼ����������ʷ�Ӧ�����ʵ���֮�ȵ��ڻ�ѧ������֮�ȵ�֪��x��y=1��2��
��3�����ݣ�2����֪������Ӧ�������������ģ���ѹǿ���
��4��l0min��ѧ��Ӧ���ʼӿ���ֱ�����ﻯѧƽ��״̬����Ӧ��������Ϊ���ºͼӴ�������ѡ�ܢޡ�16min���ǻ�ѧƽ�������ƶ����������Ӧ�Ƿ��ȷ�Ӧ���ʷ�Ӧ�������������£���ѡ�ܡ�
��5����ѧƽ�ⳣ���������������֮�����Է�Ӧ�����֮�������������K1>K2��
���������⿼�鷴Ӧ���ʡ�ƽ�ⳣ�����㡢ƽ���ƶ��ȣ��Ѷ��еȣ�����ѧ���Ի���֪ʶ�����ճ̶ȡ�

��ϰ��ϵ�д�
�����Ŀ

 N
N CO(g)+3H2(g) ��H=+206.0kJ?mol��1
CO(g)+3H2(g) ��H=+206.0kJ?mol��1

 2C(g)������2s����C��Ũ��Ϊ0.6mol��L-1���������м���˵����
2C(g)������2s����C��Ũ��Ϊ0.6mol��L-1���������м���˵���� 4NO(g)+6H2O(g)
4NO(g)+6H2O(g) CH3OH(g) + H2O(g) ��H����187.4 kJ/mol��3000Cʱ�ĺ����ܱ������У���C(CO2) = 1.00 mol.L-1 C(H2) =" 1.60" mol.L-1��ʼ��Ӧ���������ͼ��ʾ���ش��������⣺
CH3OH(g) + H2O(g) ��H����187.4 kJ/mol��3000Cʱ�ĺ����ܱ������У���C(CO2) = 1.00 mol.L-1 C(H2) =" 1.60" mol.L-1��ʼ��Ӧ���������ͼ��ʾ���ش��������⣺
 4NO(g)��6H2O(g)��H����1025 kJ/mol������Ӧ����ʼ���ʵ�����ͬ�����й��ڸ÷�Ӧ��ʾ��ͼ����ȷ����
4NO(g)��6H2O(g)��H����1025 kJ/mol������Ӧ����ʼ���ʵ�����ͬ�����й��ڸ÷�Ӧ��ʾ��ͼ����ȷ����



 NH4++NH2����NH4+��ƽ��Ũ��Ϊ1��10��15mol��L��1������˵���������(�� ��)
NH4++NH2����NH4+��ƽ��Ũ��Ϊ1��10��15mol��L��1������˵���������(�� ��)
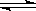 2SO3 (g) ����H��0
2SO3 (g) ����H��0