��Ŀ����
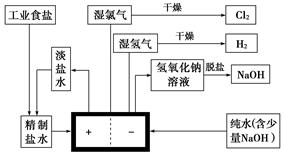
��֪��ҵʳ�κ�Ca2+��Mg2+��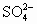 ���������ӣ�Ҫ�뾫���ɾ��Σ���ش��������⣺
���������ӣ�Ҫ�뾫���ɾ��Σ���ش��������⣺
(1)Ϊ����Ч��ȥCa2+��Mg2+��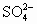 �������Լ��ĺ���˳��Ϊ��________��
�������Լ��ĺ���˳��Ϊ��________��
A���ȼ�NaOH�����Na2CO3���ټӺ�Ba2+���Լ�
B���ȼ�NaOH����Ӻ�Ba2+���Լ����ټ�Na2CO3
C���ȼӺ�Ba2+���Լ������NaOH���ټ�Na2CO3
(2)��ȥ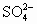 ���������Ӻ�Ba2+���Լ������Լ�������
���������Ӻ�Ba2+���Լ������Լ�������
[����]
A��Ba(OH)2
B��Ba(NO3)2
C��BaCl2
(3)���ƹ����з�����Ӧ�����ӷ���ʽΪ��________��________��________��
�𰸣�
������
������
|
����(1)BC ����(2)AC ����(3)Ba2+�� �������⣺Ҫ��NaCl�е�Ca2+��Mg2+�� |

��ϰ��ϵ�д�
�����Ŀ
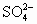 ��BaSO4��,Ca2+��
��BaSO4��,Ca2+��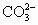 ��CaCO3��,Mg2+��2OH����Mg(OH)2��
��CaCO3��,Mg2+��2OH����Mg(OH)2��
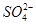 �����ϸߣ��������ӱ��Լ���ȥ
�����ϸߣ��������ӱ��Լ���ȥ
 ���ӣ��������г�������˳���ǣ�����ţ� ��
���ӣ��������г�������˳���ǣ�����ţ� ��