��Ŀ����
����������ȷ����
- A.�ϳɰ����������н�NH3Һ�����룬�ɼӿ�����Ӧ���ʣ����N2��H2��ת����
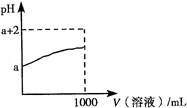
- B.��1 molBa(OH)2��ϡ��Һ�ͺ�1 molH2SO4��ϡ��Һ��Ӧ�ͷ�����a KJ�����ʾ�÷�Ӧ�к��ȵ��Ȼ�ѧ��Ӧ����ʽΪ��OH-(aq)+H+(aq)��H2O(l)����H��-a KJ/mol
- C.��ⱥ��ʳ��ˮ���ռ�������ӽ���Ĥ�����ɷ�ֹ�����Ҳ�����Cl2����������
- D.��⾫��ͭʱ��ͬһʱ���������ܽ�ͭ����������������ͭ������С
D

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
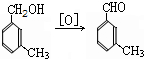
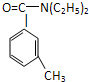 ���ð�������DEET����һ�ֶ��˰�ȫ�����Ը�����ҩ�Ե��������ü�����ṹ��ʽΪ����֪��RCOOH
���ð�������DEET����һ�ֶ��˰�ȫ�����Ը�����ҩ�Ե��������ü�����ṹ��ʽΪ����֪��RCOOH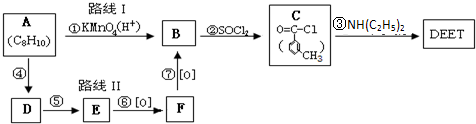
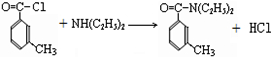
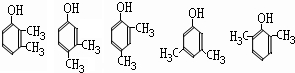 ����д2�֣�
����д2�֣�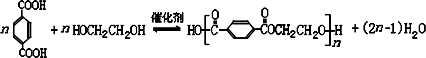
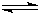 NH3?H2O+H+
NH3?H2O+H+ ��2010?��������ģ��X��Y��Z��W��Ϊ����10���ӵ���������X��Y��ZΪ���ӣ�WΪ���ӣ���X��Z�����к��еĹ��õ��Ӷ���֮��Ϊ3��4��
��2010?��������ģ��X��Y��Z��W��Ϊ����10���ӵ���������X��Y��ZΪ���ӣ�WΪ���ӣ���X��Z�����к��еĹ��õ��Ӷ���֮��Ϊ3��4��