��Ŀ����
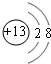
J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ����������ϼ���������ϼ۵ľ���ֵ��ȣ�M�ǵؿ��к�����������Ԫ�صķǽ���Ԫ�أ�L�������̬�⻯�����ʹʪ��ĺ�ɫʯ����ֽ������
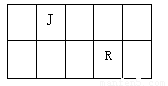
��1��M��ԭ�ӽṹʾ��ͼΪ________ ��Ԫ��R�����ڱ���λ�ڵ�_____���ڣ���_____��, Ԫ��T����������Ӧˮ���ﻯѧʽ��________��
��2��д��J�����γɵ�һ������������ĵ���ʽ____________�����й��ۼ���______����Լ���Ǽ��Լ�����
��3��д����˵��T�ǽ����Ա�Rǿ�ķ�Ӧ�Ļ�ѧ����ʽ___________________________
��4�������ӹ�ҵ�У���ˮ��Һ������ʴ��H2O2 �����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ______________________________________
��5��0.2mol��M�γɵ���̬�⻯����O2����ȫȼ�գ�����һ�ֹ����Һ̬ˮ��298Kʱ�ų�����a kJ �� �÷�Ӧ���Ȼ�ѧ����ʽΪ_____________________________________________
(15��)��1��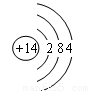 ��������A��HClO 4
��������A��HClO 4
��2��CO2����ʽ�ԣ����Լ�
��3��Cl2+H2S��S+2HCl (������������)
��4��2NH3��H2O+3H2O2��N2��+8H2O��2NH3+3H2O2��N2��+6H2O
��5��SiH4 (g)��2O2(g)��SiO2 (s)��2 H2O (l) ��H����5akJ/mol������ʽ��3�֣�����ÿ��1�֣�
����������

 ��2010?������J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
��2010?������J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
 Al��OH��3+3HCl
Al��OH��3+3HCl
 J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
 J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�������JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�������JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�