��Ŀ����
X��Y��Z�dz���Ԫ�صĵ��ʣ�U��V�ǻ�������������·�Ӧ(ʽ�и����ʵĻ�ѧ�������ͷ�Ӧ����������ȥ)
��X��U��V��Y������X��Z��V������Y��Z��U
(1)д������������Ӧ�Ļ������ͣ���ʽ��________����ʽ��________��
(2)�оٷ��Ϣ�ʽ��4����ͬ���͵�ʵ����д����Ӧ����ʽ����������ӷ�Ӧ���������ӷ���ʽ��ʾ��
________��________��________��________��
(3)��(2)������ʵ���У�Ҳ���Ϸ�Ӧ�ں͢۵ķ�Ӧ��________��
�𰸣�
������
������
|
����(1)�û���Ӧ,���Ϸ�Ӧ ����(2)H2��CuO ����(3)H2��CuO |

��ϰ��ϵ�д�
�����Ŀ
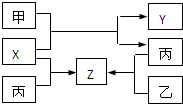 ��2009?��ģ�⣩��ͼ��ʾ���ס��ҡ��������ֳ������ʣ�X��Y��Z�dz������������֮��������ת����ϵ��
��2009?��ģ�⣩��ͼ��ʾ���ס��ҡ��������ֳ������ʣ�X��Y��Z�dz������������֮��������ת����ϵ��