��Ŀ����
����˵�����ʾ������ȷ����
[ ]
A����ϡ��Һ�У�H+��aq��+ OH-��aq��= H2O��l�� ��H=��57.3 kJ��mol-1��������0.5mol H2SO4��Ũ�����뺬1mol NaOH����Һ��ϣ��ų����ȴ���57.3kJ
B����C��ʯī��= C�����ʯ�� ��H=+1.90 kJ/mol��֪���ʯ��ʯī�ȶ�
C����ͬ�¶��£���pH=5��NH4Cl��Һ����pH=4��ϡ��������ˮ�������c(H+)����<��
D��pH��5.6��CH3COOH��CH3COONa�����Һ�У�c(Na+) > c(CH3COO-)
B����C��ʯī��= C�����ʯ�� ��H=+1.90 kJ/mol��֪���ʯ��ʯī�ȶ�
C����ͬ�¶��£���pH=5��NH4Cl��Һ����pH=4��ϡ��������ˮ�������c(H+)����<��
D��pH��5.6��CH3COOH��CH3COONa�����Һ�У�c(Na+) > c(CH3COO-)
A

��ϰ��ϵ�д�
 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
�����Ŀ
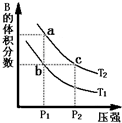 ���ڷ�ӦA��g��?2B��g����H��0�����¶�ΪT1��T2ʱ��ƽ����ϵ��B�����������ѹǿ�仯��������ͼ��ʾ���ش����и��⣮
���ڷ�ӦA��g��?2B��g����H��0�����¶�ΪT1��T2ʱ��ƽ����ϵ��B�����������ѹǿ�仯��������ͼ��ʾ���ش����и��⣮

