��Ŀ����
A��B��C��D����ѧ��ѧ�ij������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת���Ĺ�ϵ��ͼ��ʾ�����ַ�Ӧ�е�H2O����ȥ��������գ�
��1����D�����������������������;���Ľ�������
����A����������ˮ����������A�Ʊ�Ư�۵Ļ�ѧ����ʽ��______����C�Ļ�ѧʽ��______������C����Һʱ�ɼ���������______�����������ƣ�������ˮ�⣮
����A��ijǿ���ϡ��Һ����A�Ļ�ѧʽ������______��
��2����A��һ����ʹʪ��ĺ�ɫʯ����ֽ���������壬B������β��֮һ�����������ɫ����Ӧ�ٵĻ�ѧ����ʽΪ______��
��3����B������
����D��ǿ�ᣬ����ȷ��A��C�п϶��������ӵĻ�ѧʽ�ֱ���______��______��
����D��ǿ���Ӧ�ڵ����ӷ���ʽ��______��
��4����A��B��C����ɫ��Ӧ���ʻ�ɫ��D����̬�����������D������______��______���ѧʽ�����������dz���ѡ��______��______�����������Լ����ƣ���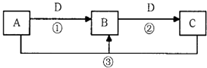
��1����D�����������������������;���Ľ�������
����A����������ˮ����������A�Ʊ�Ư�۵Ļ�ѧ����ʽ��______����C�Ļ�ѧʽ��______������C����Һʱ�ɼ���������______�����������ƣ�������ˮ�⣮
����A��ijǿ���ϡ��Һ����A�Ļ�ѧʽ������______��
��2����A��һ����ʹʪ��ĺ�ɫʯ����ֽ���������壬B������β��֮һ�����������ɫ����Ӧ�ٵĻ�ѧ����ʽΪ______��
��3����B������
����D��ǿ�ᣬ����ȷ��A��C�п϶��������ӵĻ�ѧʽ�ֱ���______��______��
����D��ǿ���Ӧ�ڵ����ӷ���ʽ��______��
��4����A��B��C����ɫ��Ӧ���ʻ�ɫ��D����̬�����������D������______��______���ѧʽ�����������dz���ѡ��______��______�����������Լ����ƣ���

��1����D�����������������������;���Ľ��������ж�ΪFe��
����A����������ˮ������֤��ΪCl2������A�Ʊ�Ư�۵Ļ�ѧ����ʽ���������������Ʒ�Ӧ�����Ȼ��ơ�������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O
��CΪFeCl2������FeCl2����Һʱ�ɼ�������������Է�ֹ�������ӵ�ˮ�⣬�ʴ�Ϊ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��FeCl2 �����
����A��ijǿ���ϡ��Һ��ʵ������ת������Ҫ�������������ᣬ�ж�ΪHNO3���ʴ�Ϊ��HNO3��
��2����A��һ����ʹʪ��ĺ�ɫʯ����ֽ���������壬�ж�AΪ��������ΪNH3��B������β��֮һ�����������ɫ��֤��DΪO2����Ӧ���ǰ����Ĵ�������Ӧ����һ��������ˮ����ѧ����ʽΪ��4NH3+5O2
4NO+6H2O���ʴ�Ϊ��4NH3+5O2
4NO+6H2O��
��3����B�����Կ���������������������������
����D��ǿ�ᣬBΪ���� ��������ΪAl��OH��3������ȷ��A��C�п϶��������ӵĻ�ѧʽ�ֱ��ǣ�AlO2-��Al3+���ʴ�Ϊ��AlO2-��Al3+��
����D��ǿ�BΪ������������ΪAl��OH��3��AΪAl3+��Ӧ��������������ǿ�Ӧ����Ӧ�����ӷ���ʽ�ǣ�Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��4����A��B��C����ɫ��Ӧ���ʻ�ɫ��֤��������Ԫ�أ�D����̬����������ʵ�������仯��D������CO2��SO2���������ʼ�������ø��������Һ��Ʒ����Һ��
�ʴ�Ϊ��Ʒ����Һ����ˮ����������Һ��
����A����������ˮ������֤��ΪCl2������A�Ʊ�Ư�۵Ļ�ѧ����ʽ���������������Ʒ�Ӧ�����Ȼ��ơ�������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O
��CΪFeCl2������FeCl2����Һʱ�ɼ�������������Է�ֹ�������ӵ�ˮ�⣬�ʴ�Ϊ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��FeCl2 �����
����A��ijǿ���ϡ��Һ��ʵ������ת������Ҫ�������������ᣬ�ж�ΪHNO3���ʴ�Ϊ��HNO3��
��2����A��һ����ʹʪ��ĺ�ɫʯ����ֽ���������壬�ж�AΪ��������ΪNH3��B������β��֮һ�����������ɫ��֤��DΪO2����Ӧ���ǰ����Ĵ�������Ӧ����һ��������ˮ����ѧ����ʽΪ��4NH3+5O2
| ||
| �� |
| ||
| �� |
��3����B�����Կ���������������������������
����D��ǿ�ᣬBΪ���� ��������ΪAl��OH��3������ȷ��A��C�п϶��������ӵĻ�ѧʽ�ֱ��ǣ�AlO2-��Al3+���ʴ�Ϊ��AlO2-��Al3+��
����D��ǿ�BΪ������������ΪAl��OH��3��AΪAl3+��Ӧ��������������ǿ�Ӧ����Ӧ�����ӷ���ʽ�ǣ�Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��4����A��B��C����ɫ��Ӧ���ʻ�ɫ��֤��������Ԫ�أ�D����̬����������ʵ�������仯��D������CO2��SO2���������ʼ�������ø��������Һ��Ʒ����Һ��
�ʴ�Ϊ��Ʒ����Һ����ˮ����������Һ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
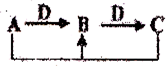 A��B��C��D����ѧ��ѧ�������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת����ϵ��ͼ�������ж���ȷ���ǣ�������
A��B��C��D����ѧ��ѧ�������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת����ϵ��ͼ�������ж���ȷ���ǣ�������| A����DΪH2O��AΪ̼����A��C��Ӧ����1 mol Bת�Ƶĵ�����ΪNA | ||
| B����DΪFe��AΪCI2����B��Һ���ɺ�ɵõ�������B���� | ||
| C����DΪO2��AΪ�������壬������ת��ֻ�漰����������ԭ��Ӧ | ||
D����DΪHCl��AΪNa2CO3����B��Һ��c��Na+��=c��HCO3-��+2c��CO
|
 ��֪A��B��C��D����ѧ��ѧ�г��������ֲ�ͬ��������֮�������ͼ��ʾ��ת����ϵ��
��֪A��B��C��D����ѧ��ѧ�г��������ֲ�ͬ��������֮�������ͼ��ʾ��ת����ϵ��

 ��֪A��B��C��D����ѧ��ѧ�г��������ֲ�ͬ���ӣ����Ҿ���10���ӵ����ӣ�����֮�������ͼ��ʾ��ת����ϵ��
��֪A��B��C��D����ѧ��ѧ�г��������ֲ�ͬ���ӣ����Ҿ���10���ӵ����ӣ�����֮�������ͼ��ʾ��ת����ϵ��
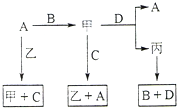 A��B��C��D ����ѧ��ѧ���������ֵ��ʣ��ס��ҡ���Ϊ���ֻ�������ڳ�����Ϊ��ɫ��ζ��Һ�壬�������ʼ�ת����ϵ��ͼ������Ӧ��������ȥ��������գ�
A��B��C��D ����ѧ��ѧ���������ֵ��ʣ��ס��ҡ���Ϊ���ֻ�������ڳ�����Ϊ��ɫ��ζ��Һ�壬�������ʼ�ת����ϵ��ͼ������Ӧ��������ȥ��������գ�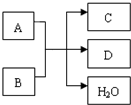 A��B��C��D����ѧ��ѧ���������ʣ�����֮�������ͼ��ʾ��ת����ϵ���밴Ҫ����գ�
A��B��C��D����ѧ��ѧ���������ʣ�����֮�������ͼ��ʾ��ת����ϵ���밴Ҫ����գ�