��Ŀ����
����Ŀ����ҵ�ϳ�����ʯ����Ҫ��CaCO3��Ϊԭ�ϣ������¹��������Ʊ�ij�����͵ij�������12CaO��7Al2O3��

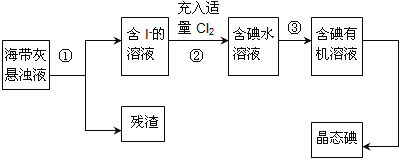
��1������ʵ�����н��С����բIJ������ɽ���Ʒ����_____________�����������ƣ��м��ȣ���������������_____________��
��2���������������У�CaO����ˮ��Ӧ����Ca(OH)2��д��Ca(OH)2��NH4NO3��Һ��Ӧ�Ļ�ѧ����ʽ��_____________�����顰��������������NH3���ɵķ�����___________��
��3����ҺX����������ѭ�����ã�����������Ҫ���ʵĻ�ѧʽΪ______��
��4������������ʱ������ӦΪ��12CaCO3��7Al2O3![]() 12CaO��7Al2O3+12CO2�����ܷ�ֱ���ð���ʯ���д˲���Ӧ����˵������_____��
12CaO��7Al2O3+12CO2�����ܷ�ֱ���ð���ʯ���д˲���Ӧ����˵������_____��
���𰸡���� ���� Ca(OH)2��2NH4NO3��Ca(NO3)2��2NH3����2H2O ��ʪ��ĺ�ɫʯ����ֽ�������壬��ֽ������������պ��Ũ����IJ������������壬�а������� NH4NO3 ���ܣ�����ʯ��Ҫ�ɷ�ΪCaCO3�����Ժ��������ɷ֣���ֱ��ʹ�ð���ʯ���������բ������Ʒ���Ȳ���
��������
����ʯ������Ҫ������Ӧ��CaCO3![]() CaO+CO2�����ڶͷ��м���������NH4NO3��Һ����CaO��Ӧ����Ca(NO3)2��NH3H2O���ʹ��˺���Һ�к�Ca(NO3)2��NH3H2O����CO2��NH3ͨ����ҺI�к�����Ӧ��Ca(NO3)2+2NH3+CO2+H2O=CaCO3��+2NH4NO3�����˵õ�̼��ơ�̼��ƺ��������������1500��C�õ�12CaO7Al2O3���Դ˽����⡣
CaO+CO2�����ڶͷ��м���������NH4NO3��Һ����CaO��Ӧ����Ca(NO3)2��NH3H2O���ʹ��˺���Һ�к�Ca(NO3)2��NH3H2O����CO2��NH3ͨ����ҺI�к�����Ӧ��Ca(NO3)2+2NH3+CO2+H2O=CaCO3��+2NH4NO3�����˵õ�̼��ơ�̼��ƺ��������������1500��C�õ�12CaO7Al2O3���Դ˽����⡣
(1)���չ��壬Ӧ�������н��У����������ڷ�������Һ�壬ӦΪ���˲������ʴ�Ϊ����������ˣ�
(2)Ca(OH)2��NH4NO3��Һ��Ӧ��������ƺͰ�������Ӧ�Ļ�ѧ����ʽΪCa(OH)2+2NH4NO3=Ca(NO3)2+2NH3��+2H2O������Ϊ�������壬���ú�ɫʯ����ֽ���飬Ҳ����Ũ������飬�����ǽ�ʪ��ĺ�ɫʯ����ֽ�������壬��ֽ������(����պ��Ũ����IJ������������壬�а������ɡ�)���ʴ�Ϊ��Ca(OH)2+2NH4NO3=Ca(NO3)2+2NH3��+2H2O����ʪ��ĺ�ɫʯ����ֽ�������壬��ֽ������(����պ��Ũ����IJ������������壬�а������ɡ�)��
(3)��CO2��NH3ͨ����ҺI�к�����Ӧ��Ca(NO3)2+2NH3+CO2+H2O=CaCO3��+2NH4NO3����ѭ�����õ�ΪNH4NO3���ʴ�Ϊ��NH4NO3��
(4)����ʯ��Ҫ�ɷ�ΪCaCO3�����Ժ��������ɷ֣���ֱ��ʹ�ð���ʯ���������������Ʒ���Ȳ�������ֱ�����հ���ʯ���ʴ�Ϊ�����ܣ�����ʯ��Ҫ�ɷ�ΪCaCO3�����Ժ��������ɷ֣���ֱ��ʹ�ð���ʯ���������������Ʒ���Ȳ��ߡ�

����Ŀ���л���A���������Ƿ��͵õ���Ҳ���Դ�ţ������ȡ��������AΪ��ɫճ��Һ�壬������ˮ��Ϊ�о�A�������ṹ������������ʵ�飺
ʵ�鲽�� | ���ͻ�ʵ����� |
��ȡA9.0g������ʹ�������������ܶ�����ͬ������H2��45�� | ��ͨ��������գ� ��1��A����Է�������Ϊ__�� |
��9.0gA��������O2�г��ȼ�գ���ʹ��������λ���ͨ��Ũ���ᡢ��ʯ�ң��������߷ֱ�����5.4g��13.2g | ��2��A�ķ���ʽΪ__�� |
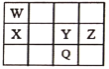
��ȡA9.0g����������NaHCO3��ĩ��Ӧ������2.24LCO2����״�������������������Ʒ�Ӧ������2.24LH2����״������ | ��3���ýṹ��ʽ��ʾA�к��еĹ�����__��__�� |
A�ĺ˴Ź���������ͼ��
| ��4��A�к���__����ԭ�ӡ� ����������A�Ľṹ��ʽΪ__�� |