��Ŀ����
12�� ����ͼ����ʯī���缫�ĵ����У��׳���Ϊ500mL��ijһ���ʵ���ɫ��Һ���ҳ���Ϊ500mLϡ���ᣬ�պ�K1���Ͽ�K2���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�����Һ�е�ԭ��������ȫ��������ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫��������1.6g����ش��������⣺
����ͼ����ʯī���缫�ĵ����У��׳���Ϊ500mL��ijһ���ʵ���ɫ��Һ���ҳ���Ϊ500mLϡ���ᣬ�պ�K1���Ͽ�K2���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�����Һ�е�ԭ��������ȫ��������ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫��������1.6g����ش��������⣺��1���������У��ҳ�C�缫������Ӧ�ĵ缫��Ӧʽ2H++2e-=H2����
��2���׳ص��ʱ��Ӧ�����ӷ���ʽ2Cu2++2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2Cu+O2��+4H+��
��3���׳ص�����Һ��pH=1��Ҫʹ������Һ�ָ������ǰ��״̬���������CuO��������Ϊ2g����������ǰ����Һ��������䣩
��4���������ٽ�K1�Ͽ����պ�K2��������ָ�뷢��ƫת����D�缫������Ӧ�ĵ缫��ӦʽO2+4e-+4H+=2H2O��
���� ��1��A�缫�����к�ɫ�Ĺ�̬�������ɣ�����A�缫��������B�缫����ɫ�������ɣ�����B������������C���������õ缫������������������D�缫���������ü��ϲ�����������������q��������p�Ǹ��������ݵ缫�����ӵķŵ������д�缫��Ӧʽ��
��2���������ͭ�������ᡢͭ���������ݴ˻ش�
��3���������ɵ�ͭ�����������������������������pH������ʸ�ԭ�ķ�������ʲô��ʲô��
��4���ٽ�K1�Ͽ����պ�K2��������ָ�뷢��ƫת���γ�����ȼ�ϵ�أ�ͨ�������������������õ��ӵĻ�ԭ��Ӧ��
��� �⣺A�缫�����к�ɫ�Ĺ�̬�������ɣ�����A�缫��������B�缫����ɫ�������ɣ�����B������������C���������õ缫������������������D�缫���������ü��ϲ�����������������q��������p�Ǹ�����
��1���������У��ҳ�C�缫���������õ缫������Ӧ�ĵ缫��ӦʽΪ��2H++2e-=H2�����ʴ�Ϊ��2H++2e-=H2����
��2���������ͭ�������ᡢͭ���������׳ص��ʱ��Ӧ�����ӷ���ʽΪ��2Cu2++2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2Cu+O2��+4H+��
�ʴ�Ϊ��2Cu2++2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2Cu+O2��+4H+��
��3��ȡ��A�缫��ϴ�ӡ�����������缫��������1.6g�������ɽ���ͭ�����ʵ�����0.025mol������2Cu2++2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2Cu+O2��+4H+��
���������������ӵ����ʵ�����0.05mol������������Ũ����$\frac{0.05mol}{0.5L}$=0.1mol/L��pH=1������ʸ�ԭ�ķ�������ʲô��ʲô��Ҫʹ������Һ�ָ������ǰ��״̬������ӣ�0.025mol������ͭ��������2g���ʴ�Ϊ��1��CuO��2��
��4���ٽ�K1�Ͽ����պ�K2��������ָ�뷢��ƫת���γ�����ȼ�ϵ�أ�ͨ���������������õ缫�Ϸ����õ��ӵĻ�ԭ��Ӧ��O2+4e-+4H+=2H2O��
�ʴ�Ϊ��O2+4e-+4H+=2H2O��
���� �����ۺϿ���ѧ��ԭ��غ͵��صĹ���ԭ���Լ��缫��Ӧʽ����д�ͼ���֪ʶ�������ۺ�֪ʶ�Ŀ��飬֪ʶ�Ĺ��ɺ������ǹؼ����ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | �����£�pH=9��NaHA��Һ��c��Na+����c��HA-����c��A2-��c��H2A�� | |
| B�� | Na2CO3��Һ��c��H+��-c��OH-��=c��HCO3-��+2c��CO32-��-c��Na+�� | |
| C�� | ��NaOH��Һ�е���HCOOH��Һ����Һ�Լ��ԣ�c��HCOO-����c��OH-����c��H+�� | |
| D�� | Ũ�Ⱦ�Ϊ0.1mol•L-1HF��Һ��0.1mol•L-1KF��Һ�������ϣ�c��F-��+c��HF��=0.2mol•L-1 |
| A�� | ��ij����ͨ����ˮ�У���ˮ��ɫ��ȥ��������һ������ϩ | |
| B�� | ��������ʱ�¶ȼ�ˮ�����ڷ�ӦҺ�� | |
| C�� | ʵ��������ϩʱ�¶ȼ�ˮ�����ڷ�ӦҺ�� | |
| D�� | �����������е���Ԫ��ʱ����������������NaOH��Һ��Ϲ��ȣ���ַ�Ӧ����ȴ�μ�AgNO3��Һ |
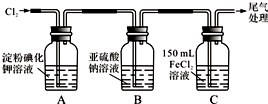
��1��ͨ��������A�е���������Һ����ɫ��Cװ���з�����Ӧ�����ӷ���ʽΪ��2Fe2++Cl2=2Fe3++2Cl-��
��2����ͨ������һ��ʱ���Bƿ����Һ����һ����SO32-������SO42-��������鷽��������Bƿ��Һ��Cl-��SO42-�Ĵ��ڣ�
| ʵ �� �� �� | Ԥ������ͽ��� |
| ����1��ȡ����Bƿ����Һ��һ�ɾ��Թ��У��μӹ���ϡ�����������BaCl2��Һ���� | ��������ɫ��������Bƿ��Һ�д���SO42-�� |
����2����ȡ����Bƿ����Һ���Թ�һ�ɾ��Թ��У��μӹ�����2mol/L HNO3��l mol/L Ba��NO3��2��Һ�������ã� | ������ɫ������ |
| ����3��ȡ����2���Թ��е��ϲ���Һ��һ�ɾ��Թ��У��μ�01mol/L AgNO3��Һ���� | ��������ɫ��������Bƿ��Һ�д���Cl-�� |
��3��Ϊȷ�ⶨͨ������һ��ʱ���Cƿ��ʣ��FeCl2�����ʵ�����ʵ�����£�
������250mL ��Һ����Cƿ��ȫ����Һȡ��ʢ��250mL����ƿ�У���ȷ���Ƴ�250mL��Һ��
ȷ��Cƿ�е���Һȫ��ȡ������������ʧ��������еIJ����ǽ�Cƿ�е���Һת�Ƶ�����ƿ������������ˮϴ��Cƿ2��3�Σ���ת������ƿ��
�ڵζ���ȷ��ȡ25.00mL������Һ����ƿ�У���0.20mol/L KMnO4��Һװ����ʽ�ζ��ܣ��ζ����յ㣬��¼���ݣ��ظ��ζ�2�Σ�ƽ������KMnO4��ҺV mL������Ӧ����ʽ��Fe2++MnO4-+H+-Fe3++Mn2++H2O��δ��ƽ��
�ۼ��㣺Cƿ��ʣ��FeCl2�����ʵ�����n��FeCl2��=0.01Vmol��
| A�� | ���ӻ������п��ܺ����ۼ� | B�� | ���ۻ������п��ܺ����Ӽ� | ||
| C�� | ���ӻ������в������ۼ� | D�� | ���ۻ������в������Ӽ� |
| A�� | �������õ��Ӷ���ĿΪ$\frac{a}{7+1}$NA | B�� | ����̼�����ĿΪ$\frac{a{N}_{A}}{7}$ | ||
| C�� | ȼ��ʱ���ĵ�O2һ����$\frac{33.6a}{14}$ L | D�� | ����ԭ������Ϊ$\frac{a{N}_{A}}{14}$ |



 $��_{OH-}^{��CH_{3}��_{2}SO_{4}}$
$��_{OH-}^{��CH_{3}��_{2}SO_{4}}$ $\stackrel{ŨHI}{��}$
$\stackrel{ŨHI}{��}$
 ��
�� +Cl2$\stackrel{����}{��}$
+Cl2$\stackrel{����}{��}$ +HCl����Ӧ����Ϊȡ����Ӧ��
+HCl����Ӧ����Ϊȡ����Ӧ�� ��
�� ����
���� ����
���� $\stackrel{��Ӧ����1}{��}$I$��_{OH-}^{��CH_{3}��_{2}SO_{4}}$J$\stackrel{��Ӧ����2}{��}$K$\stackrel{��Ӧ����3}{��}$
$\stackrel{��Ӧ����1}{��}$I$��_{OH-}^{��CH_{3}��_{2}SO_{4}}$J$\stackrel{��Ӧ����2}{��}$K$\stackrel{��Ӧ����3}{��}$ ��Ӧ����1���õ��Լ�Ϊ��CH3��3CCl/AlCl3��K�Ľṹ��ʽΪ
��Ӧ����1���õ��Լ�Ϊ��CH3��3CCl/AlCl3��K�Ľṹ��ʽΪ ����Ӧ����3���õ��Լ�ΪŨHI��
����Ӧ����3���õ��Լ�ΪŨHI��
