��Ŀ����
4����̼���ƣ�2Na2CO3•3H2O2����һ�ּ�ϴ�ӡ�Ư�ס�ɱ����һ�����ϵƯ����ij��ȤС���Ʊ���̼���Ƶ�ʵ�鷽��ͼ1��װ��ʾ��ͼ������ͼ2��
��֪������Ӧ 2Na2CO3��aq��+3H2O2��aq��?2Na2CO3•3H2O2 ��s����H��0
����Ӧ 2H2O2=2H2O+O2��
50��ʱ2Na2CO3•3H2O2��s�� ��ʼ�ֽ�
��ش��������⣺
��1������ٵĹؼ��ǿ����¶ȣ�ԭ�����Ʊ���̼�����Ƿ��ȷ�Ӧ����ֹ�ֽ⣬���ʩ����ˮԡ����������ͻ����μ�H2O2��Һ��
��2������ҺX�м�������NaCl�����������̼���ƣ�ԭ��������������Ũ�ȡ����Ͳ�Ʒ���ܽ�ȣ��������ã���
��3���������ѡ����ˮ�Ҵ�ϴ�Ӳ�Ʒ��Ŀ����ϴȥˮ�ݣ����ڸ��
��4�����������У��������̼����ʧЧ����BD��
A��NaHCO3 B��MnO2 C��Na2SiO3 D��Na2SO3
��5����̼���Ʋ�Ʒ��������������̼���ƣ������������ⶨ��̼���Ƶ�������������������裺ȡ��Ʒ�ܽ������BaCl2��Һ�����˹��ˡ�ϴ��ϴ�ӡ��������
����������Ҫֱ�Ӳⶨ���������У���Ʒ������m1g������������m2g������ĸ��ʾ��ע���京�壩����Ʒ�й�̼�������������ı���ʽΪ��$\frac{314��{m}_{1}-\frac{106{m}_{2}}{197}��}{102{m}_{1}}$��
���� ˫��ˮ��̼���ƻ�Ͽ����¶ȷ�����Ӧ2Na2CO3 ��aq��+3H2O2 ��aq��?2Na2CO3•3H2O2��s�������ù��˵õ�����2Na2CO3•3H2O2��������ϴ�ӡ�����õ��ϴ�����2Na2CO3•3H2O2��
��1���¶ȹ���ʱ��̼���Ʒֽ������ʹ��ͣ���Ӧ�¶Ƚϵͣ������¶ȿ�Ǩ�Ʊ������ᷴӦ���¶ȿ��Ʒ����������Խ�����ȴ���÷�Ӧ���ʼ�С�������μ�H2O2��Һ��
��2����ҺX�к����ܽ�Ĺ�̼���ƣ�������Ũ��Խ��̼���Ƶ��ܽ��ԽС��
��3����̼�����������Ҵ���ˮ���Ҵ����ܣ�
��4����̼�����൱�ڴ��ᾧ˫��ˮ��̼���ƣ�����˫��ˮ�����ʣ���������ԭ�������״ٽ���̼���Ʒ�Ӧ������ʧЧ��
��5���������ⶨ��̼��������������Ҫ������Ʒ�����ͳ����������з��̼����̼���Ƶ���������������õ���̼��������������
��� �⣺˫��ˮ��̼���ƻ�Ͽ����¶ȷ�����Ӧ2Na2CO3 ��aq��+3H2O2 ��aq��?2Na2CO3•3H2O2��s�������ù��˵õ�����2Na2CO3•3H2O2��������ϴ�ӡ�����õ��ϴ�����2Na2CO3•3H2O2��
��1���Ʊ���̼�����Ƿ��ȷ�Ӧ����Ӧ�����зų�������ʹ�¶����ߣ��¶ȹ��ᵼ�¹�̼���Ʒֽ⣬�Ӷ���������ʣ�
��Ӧ�¶Ƚϵͣ������¶ȿ�Ǩ�Ʊ������ᷴӦ���¶ȿ��Ʒ�������ˮԡ��������Ҳ���Կ����¶ȣ����Ի����ô�������ķ������÷�Ӧ���ʼ�С�����¶ȣ����Կ��û����μ�H2O2��Һ�ķ�����
�ʴ�Ϊ���Ʊ���̼�����Ƿ��ȷ�Ӧ����ֹ�ֽ⣻��ˮԡ���������裻�����μ�H2O2��Һ��
��2�������Ȼ��ƹ��壬������Ũ���������˹�̼���Ƶ��ܽ�ȣ�����������̼���ƣ�
�ʴ�Ϊ������������Ũ�ȡ����Ͳ�Ʒ���ܽ�ȣ��������ã���
��3����ˮ�Ҵ��ܺ�ˮ���ܣ��Ҽ��ӷ�������ѡ����ˮ�Ҵ�ϴ�ӣ�������Ŀ���ǣ�ϴȥˮ�ݣ����ڸ��
�ʴ�Ϊ��ϴȥˮ�ݣ����ڸ��
��4����̼�����൱�ڴ��ᾧ˫��ˮ��̼���ƣ�����˫��ˮ�����ʣ���������ԭ�������״ٽ���̼���Ʒ�Ӧ������ʧЧ��������������˫��ˮ�Ĵ������������ƾ��л�ԭ�ԣ�̼�����ƺ����ƶ������̼���Ʒ�Ӧ�������������̼����ʧЧ���Ƕ������̺��������ƣ���ѡ��BD��
��5��ʵ�������ֱ�Ӳⶨ��������Ϊ��Ʒ������m1g������������m2g���������ʵ���=$\frac{{m}_{2}g}{197g/mol}$=$\frac{{m}_{2}}{197}$mol�����̼�������ʵ���Ϊx�����е�̼�������ʵ���Ϊy��
����̼Ԫ���غ�õ���2x+y=$\frac{{m}_{2}}{197}$��
�������ɵã�314x+106y=m1
���x=$\frac{{m}_{1}-\frac{{m}_{2}��106}{197}}{102}$
��̼������������=x=$\frac{{m}_{1}-\frac{{m}_{2}��106}{197}}{102}$mol��314g/mol��m1g=$\frac{314��{m}_{1}-\frac{106{m}_{2}}{197}��}{102{m}_{1}}$��
�ʴ�Ϊ����Ʒ������m1g������������m2g��$\frac{314��{m}_{1}-\frac{106{m}_{2}}{197}��}{102{m}_{1}}$��
���� ���⿼���Ʊ�ʵ�鷽����ƣ����ؿ�����������㼰֪ʶǨ�ơ����������������ѵ��ǹ�̼�������������ļ��㣬�������ԭ���غ��𣬣�4������֪ʶǨ�Ʒ�����ɣ���Ŀ�Ѷ��еȣ�

 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д� ��1000mL3mol•L-1NaOH��ͨ������CO2���壬��Һ���й������ӵ����ʵ����仯������ͼ��ʾ������˵����ȷ���ǣ�������
��1000mL3mol•L-1NaOH��ͨ������CO2���壬��Һ���й������ӵ����ʵ����仯������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ˮ�ĵ���̶�һֱ���� | |
| B�� | ��Һ��pH���½� | |
| C�� | M��N�����Ӧ����Һ���������ͬ | |
| D�� | CD�η�Ӧ�����ӷ���ʽΪOH-+CO2=HCO3- |
| A�� | �ձ� | B�� | ������ | C�� | ������ƿ | D�� | �Թ� |
| A�� | �ױ�����������֬���������ɿ���ȡ����Ӧ | |
| B�� | ������ˮ�����������Ƿų��������ṩ����������� | |
| C�� | ʯ���ѽ��Ŀ����Ϊ���������Һ��ȼ�ϵIJ��������� | |
| D�� | ���ͺϳ���ά����Ҫ�ɷ�����ά�� |
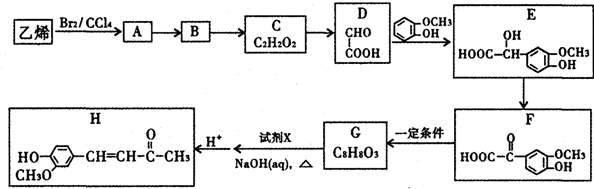
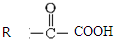 $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$ 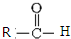 +CO2
+CO2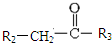 $\stackrel{NaOH��aq������}{��}$
$\stackrel{NaOH��aq������}{��}$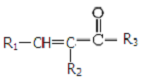 +H2O
+H2O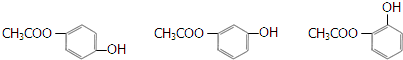 ������֮һ����
������֮һ����
 ijѧ��Ϊ����֤���ӡ����ᡢ̼�������ǿ���������ʵ��װ�ã��ش��������⣺
ijѧ��Ϊ����֤���ӡ����ᡢ̼�������ǿ���������ʵ��װ�ã��ش��������⣺ ��
��