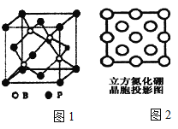
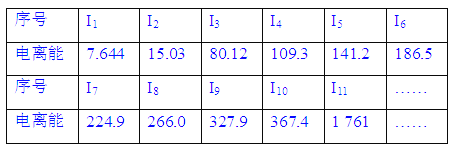
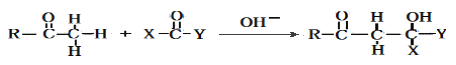��Ŀ����
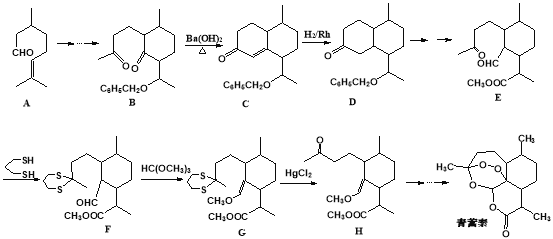
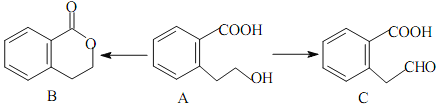
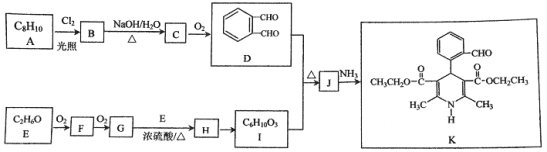
����Ŀ���л���K�����Ƹ�Ѫѹҩ�����Ҫ�м��壬���ĺϳ�·������(����ת��������ȥ)
��֪��
����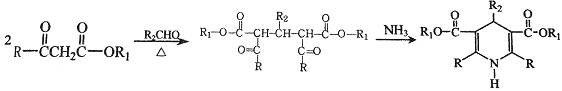
����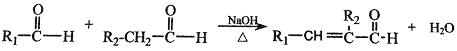 (R��R1��R2��ʾ��ԭ�ӻ�����)
(R��R1��R2��ʾ��ԭ�ӻ�����)
��1��A�Ľṹ��ʽ��__________��
��2��G��E����H�Ļ�ѧ����ʽ��__________.
��3��C��D�Ļ�ѧ����ʽ��__________.
��4��I�Ľṹ��ʽ��__________��
��5���й�J��˵���У���ȷ����__________(ѡ����ĸ)��
a������NaHCO3��Ӧb������NaOH��Ӧc����������Cu(OH)2��Ӧ
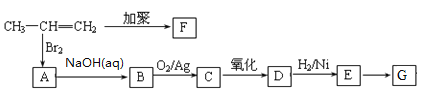
��6��K��������ת����ϵ��K![]() M(C16H15NO5Na2)��M�Ľṹ��ʽ��__________��
M(C16H15NO5Na2)��M�Ľṹ��ʽ��__________��
���𰸡���1��![]() ����2��CH3COOH+C2H5OH
����2��CH3COOH+C2H5OH ![]() CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
��3��![]() +O2
+O2 ![]()
![]() +2H2O��
+2H2O��
��4��![]() ����5��bc����6��
����5��bc����6�� ��
��
��������
������������A�ķ���ʽ��D�Ľṹ����֪AΪ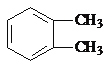 ����BΪ
����BΪ![]() ��CΪ
��CΪ![]() ��E������������������Ӧ�����E�ķ���ʽ��֪EΪC2H5OH����FΪCH3CHO��GΪCH3COOH��HΪCH3COOC2H5��I��C��Ӧ�õ�J��J�백����Ӧ�õ�K����K�Ľṹ���ƽ����Ϣ����֪JΪ
��E������������������Ӧ�����E�ķ���ʽ��֪EΪC2H5OH����FΪCH3CHO��GΪCH3COOH��HΪCH3COOC2H5��I��C��Ӧ�õ�J��J�백����Ӧ�õ�K����K�Ľṹ���ƽ����Ϣ����֪JΪ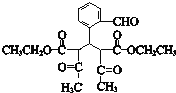 ��IΪ
��IΪ![]() ��
��
��1��A�Ľṹ��ʽ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��G��E����H�Ļ�ѧ����ʽ�ǣ�CH3COOH+C2H5OH ![]() CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+C2H5OH
CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+C2H5OH ![]() CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
��3��C��D�Ļ�ѧ����ʽ�ǣ�![]() +O2
+O2 ![]()
![]() +2H2O���ʴ�Ϊ��
+2H2O���ʴ�Ϊ��![]() +O2
+O2 ![]()
![]() +2H2O��
+2H2O��
��4��I�Ľṹ��ʽ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��5��JΪ ��a��û���Ȼ���������NaHCO3��Ӧ����a����b����������������NaOH��Ӧ����b��ȷ��c������ȩ������������Cu(OH)2��Ӧ����c��ȷ����ѡ��bc��
��a��û���Ȼ���������NaHCO3��Ӧ����a����b����������������NaOH��Ӧ����b��ȷ��c������ȩ������������Cu(OH)2��Ӧ����c��ȷ����ѡ��bc��
��6��K��������ת����ϵ��K![]() M(C16H15NO5Na2)��������Ϣ���з�Ӧ��������������ˮ�ⷴӦ����M�Ľṹ��ʽ��
M(C16H15NO5Na2)��������Ϣ���з�Ӧ��������������ˮ�ⷴӦ����M�Ľṹ��ʽ��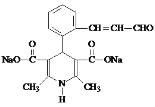 ���ʴ�Ϊ��
���ʴ�Ϊ��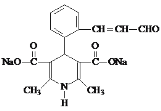 ��
��