��Ŀ����
��1��ijԪ������ϼ�Ϊ��5��ԭ������������Ϊ2���뾶��ͬ������С�ģ����������Ų�ʽΪ
_________���۵��ӹ���Ϊ_________����_________��Ԫ�ء�
��2��ijԪ��ԭ�ӵļ۵��ӹ���Ϊ4s24p1�������ڵ�_________���ڣ�_________�壬��������ϼ�Ϊ_________��Ԫ�ط�����_________��
��3��ԭ������Ϊ24��Ԫ��ԭ������_________�����Ӳ㣬_________���ܼ���_________��δ�ɶԵ��ӡ�
��4����д����2���ܲ��p�����ֻ��һ��δ�ɶԵ��ӵĻ�̬ԭ�ӵ���Χ�����Ų�ͼ���м���д������
__________________
_________���۵��ӹ���Ϊ_________����_________��Ԫ�ء�
��2��ijԪ��ԭ�ӵļ۵��ӹ���Ϊ4s24p1�������ڵ�_________���ڣ�_________�壬��������ϼ�Ϊ_________��Ԫ�ط�����_________��
��3��ԭ������Ϊ24��Ԫ��ԭ������_________�����Ӳ㣬_________���ܼ���_________��δ�ɶԵ��ӡ�
��4����д����2���ܲ��p�����ֻ��һ��δ�ɶԵ��ӵĻ�̬ԭ�ӵ���Χ�����Ų�ͼ���м���д������
__________________
���ԡ�

��ϰ��ϵ�д�
�����Ŀ
��8�֣����в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ���±���
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| T | M������2�ԳɶԵ��� |
| X | �����������Ǵ�����������2�� |
| Y | �����µ���Ϊ˫ԭ�ӷ��ӣ����⻯��ˮ��Һ�ʼ��� |
| Z | Ԫ����������ǣ�7�� |
��2��Ԫ��Y����Ԫ�ؿ��γ�����YH
 ��YH
��YH ���ӵĽṹʽΪ ��
���ӵĽṹʽΪ ����3��Ԫ��Z��T��ȣ��ǽ����Խ�ǿ����_______����Ԫ�����ƣ������б�������֤����һ��ʵ����________��
a.������Z�ĵ��ʺ�T�ĵ���״̬��ͬ
b.Z���⻯���T���⻯���ȶ�
c.һ��������Z��T�ĵ��ʶ���������������Һ��Ӧ
d.Z������H2����
e.Z��T�����������ϣ���Fe����Z���ɵĻ������м�̬����
��4��д������������ij����Ԫ����ɵķǼ��Է��ӵĽṹʽ
�� ��
��8�֣����в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ���±���
|
Ԫ�ر�� |
Ԫ�����ʻ�ԭ�ӽṹ |
|
T |
M������2�ԳɶԵ��� |
|
X |
�����������Ǵ�����������2�� |
|
Y |
�����µ���Ϊ˫ԭ�ӷ��ӣ����⻯��ˮ��Һ�ʼ��� |
|
Z |
Ԫ����������ǣ�7�� |
��1��Ԫ��T��ԭ������㹲��______�ֲ�ͬ�˶�״̬�ĵ��ӡ�Ԫ��X��һ��ͬλ�ؿɲⶨ�������������ͬλ�صķ�����_________��
��2��Ԫ��Y����Ԫ�ؿ��γ�����YH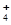 ��YH
��YH ���ӵĽṹʽΪ
��
���ӵĽṹʽΪ
��
��3��Ԫ��Z��T��ȣ��ǽ����Խ�ǿ����_______����Ԫ�����ƣ������б�������֤����һ��ʵ����________��
a.������Z�ĵ��ʺ�T�ĵ���״̬��ͬ
b.Z���⻯���T���⻯���ȶ�
c.һ��������Z��T�ĵ��ʶ���������������Һ��Ӧ
d.Z������H2����
e.Z��T�����������ϣ���Fe����Z���ɵĻ������м�̬����
��4��д������������ij����Ԫ����ɵķǼ��Է��ӵĽṹʽ
�� ��
 (NH4)2S2O8 +
H2��
(NH4)2S2O8 +
H2��
